This question is about the use of different formulae to represent chemicals.
Explain what is meant by the terms empirical formula and molecular formula, using a named alkene as an example.
Analysis of an unknown hydrocarbon, X, showed the following percentage composition by mass: C = 82.8, H = 17.2
What is the empirical formula of the unknown hydrocarbon?
(Ar: C = 12.0 , H = 1.0)
A student suggests that the structure of the unknown hydrocarbon, X, could be:
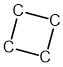
Explain, with reasons, whether the student is correct.
Unknown hydrocarbon, Y, is analysed and a student completes the following calculation to determine that the compound Y is ethane:
| C | H |
|---|---|---|
Value | 81.8% | 18.2% |
Atomic mass | 12.0 | 1.0 |
Moles | 6.817 | 18.2 |
Ratio | 1 | 2.67 → 3 |
Identify the error that the student has made and explain how they should have completed the calculation.
What extra piece of information could have told the student that their answer to part (d) was incorrect?
Was this exam question helpful?
