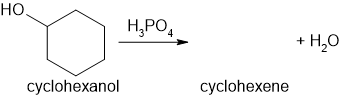
The formation of a white precipitate with excess sodium hydroxide or excess sulfuric acid is often used as a way to identify Group 2 metal ions in solution.
Explain why the sodium hydroxide test or the sulfuric acid test should not be used on their own.
Your answer should talk about the reactions of Group 2 metal ions with excess sodium hydroxide and with excess sulfuric acid.
The test for the ammonium ion is the use of litmus paper as shown in Figure 1.
Figure 1

State the error shown in Figure 1 when testing for the ammonium ion.
A student tested a solution known to contain a halide ion. It was found to produce a creamy yellow precipitate. Further testing showed that the precipitate did not dissolve.
Identify the two reagents the student used to test the solution.
Acid is used to test for the presence of the carbonate ion. The gas produced during this test has to be bubbled through limewater to prove that it is carbon dioxide.
Write an ionic equation, including state symbols, for the reaction of the carbonate ion with acid to form carbon dioxide and one other product.
Did this page help you?