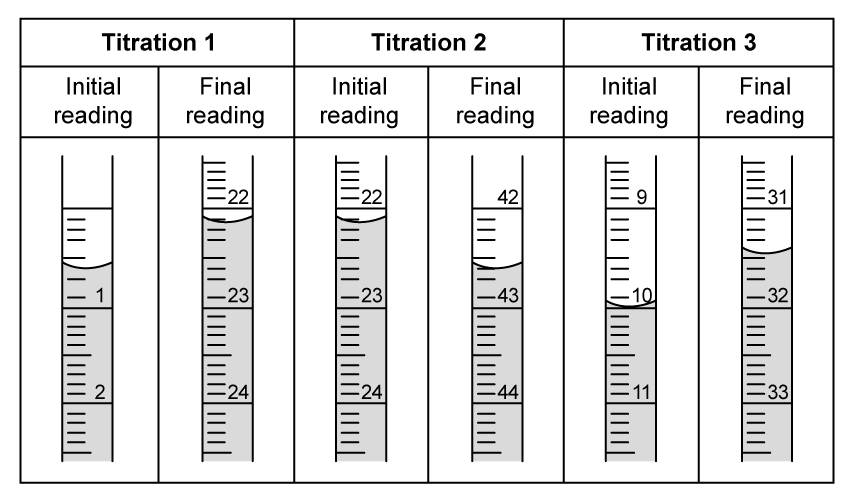
A student planned an experiment to determine the empirical formula of magnesium oxide by heating magnesium and recording the resulting change in mass.
Some of the equipment the student used is shown in the diagram below.

Suggest another piece of equipment that would be essential to find the empirical formula of magnesium oxide.
The method the student used is shown below.
Measure the mass of the crucible together with its lid
Coil a 10 cm strip of magnesium ribbon and place into the crucible
Reweigh the crucible and lid
Heat the crucible strongly using the apparatus in the diagram in part (a)
Use the tongs to lift the crucible lid slightly during heating
As it nears completion, place the crucible on a heat-proof mat
Allow the crucible to cool and reweigh the crucible and lid
i) Explain the purpose of Step 5.
[1]
ii) How could you be sure that the reaction had gone to completion?
[1]
The student obtained the following results.
Mass of crucible and lid / g | 25.68 |
Mass of magnesium, crucible and lid / g | 27.01 |
Mass of magnesium oxide, crucible and lid / g | 27.81 |
Using the results in the table above, deduce the empirical formula of magnesium oxide.
Another student performed the same experiment but tapped the residue with tongs to break it up during Step 6.
i) Suggest a reason for this.
[1]
ii) Suggest how this would affect their calculation of empirical formula.
[1]
Was this exam question helpful?