Reactions of Carbonyl Compounds (OCR A Level Chemistry A): Revision Note
Exam code: H432
Oxidation of Aldehydes
Aldehydes and ketones contain the carbonyl functional group, C=O
This is why aldehydes and ketones are also known as carbonyls
The difference between aldehydes and ketones is the groups bonded to the carbon of the carbonyl group

The carbonyl group in an aldehyde is always situated at the end of the chain
When naming aldehydes, you do not include the '1' in the name, the carbonyl carbon is always number 1 on the chain
The simplest aldehyde is methanal, HCHO, with the only carbon being that of the carbonyl group
The carbonyl group in a ketone is always situated in the middle of the chain
The simplest ketone is propan-2-one, CH3COCH3, as you need an alkyl group either side of the carbonyl carbon in a ketone
During the oxidation of a primary alcohol to an aldehyde, the apparatus must be set up to distill off the aldehyde as it is produced
Further oxidation of primary alcohols can then take place
Aldehydes can be easily oxidised to form carboxylic acids
To oxidise a primary alcohol straight to a carboxylic acid, you would heat the reaction mixture under reflux
The aldehyde would still be produced, but as it evaporates it would condense and drop back into the reaction mixture, to be further oxidised to the carboxylic acid
The oxidising agent used for all of the oxidation reactions be acidified potassium dichromate
K2Cr2O7 with sulfuric acid, H2SO4
Ketones are very resistant to being oxidised, so no further oxidation reaction will take place with secondary alcohols
This is because ketones do not have a readily available hydrogen atom, in the same way that aldehydes (or alcohols) do
An extremely strong oxidising agent would be needed for oxidation of a ketone to take place
The oxidation will likely oxidise a ketone in a destructive way, breaking a C-C bond

Nucleophilic Addition Reactions
Many of the reactions which carbonyl compounds undergo are nucleophilic addition reactions
The carbonyl group -C=O, in aldehydes and ketones is polarised
The oxygen atom is more electronegative than carbon drawing electron density towards itself
This leaves the carbon atom slightly positively charged and the oxygen atom slightly negatively charged
The carbonyl carbon is therefore susceptible to attack by a nucleophile, such as the cyanide ion
The carbonyl group here has a dipole with a delta positive carbon and a delta negative oxygen

General Mechanism with an aldehyde:

General Mechanism with a ketone:

In both reactions, the nucleophile (Nu) attacks the carbonyl carbon to form a negatively charged intermediate which quickly reacts with a proton
Addition of HCN to carbonyl compounds
The nucleophilic addition of hydrogen cyanide to carbonyl compounds is a two-step process, as shown below

In step 1, the cyanide ion attacks the carbonyl carbon to form a negatively charged intermediate
In step 2, the negatively charged oxygen atom in the reactive intermediate quickly reacts with aqueous H+ (either from HCN, water or dilute acid) to form 2-hydroxynitrile compounds,
e.g. 2-hydroxypropanenitrile
This reaction is important in organic synthesis, because it adds a carbon atom to the chain, increasing the chain length
The products of the reaction are hydroxynitriles
The nitrile group is the priority functional group so it is attached to carbon 1 and results in the suffix -nitrile
The hydroxyl group is not the priority functional group so the hydroxyl group is named using the hydroxy- prefix, rather than the -ol suffix
Reduction of Carbonyls
There are a large number of reducing agents which will reduce both an aldehyde and a ketone to an alcohol
Aldehydes are reduced to primary alcohols and ketones are reduced to secondary alcohols
Possibly the most common reducing agent for this is sodium tetrahydridoborate, NaBH4
You may also see this named as sodium borohydride in some sources
In an aqueous solution, NaBH4 generates the hydride ion nucleophile, :H-
The hydride ion will reduce a carbonyl group in an aldehyde or a ketone, but is not strong enough to reduce a C=C double bond
This is because it is attracted to the C in the C=O bond, but is repelled by the high electron density of the C=C bond
When this reaction takes place, it is an example of a nucleophilic addition reaction
Reduction Reactions
Carboxylic acid to a primary alcohol:
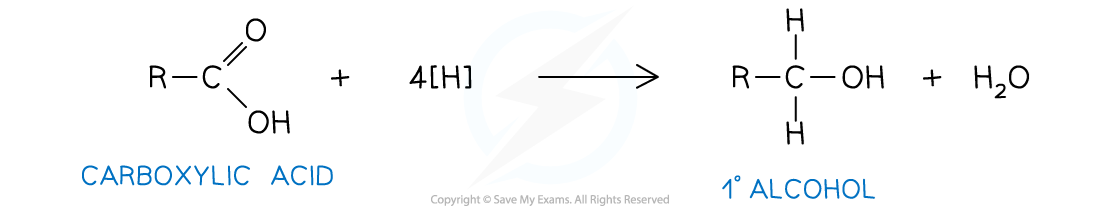
Aldehyde to a primary alcohol:

Ketone to a secondary alcohol:

Examiner Tips and Tricks
In theory the reduction of a carboxylic acid is a two stage process, from the carboxylic acid to the aldehyde and then further reduction from the aldehyde to the primary alcohol. In reality however, the reaction would really go from the carboxylic acid straight to the primary alcohol. Be careful and check the wording of the question when asked about the reduction of a carboxylic acid!

Unlock more, it's free!
Was this revision note helpful?
