Gibbs Free Energy, ΔG (OCR A Level Chemistry A) : Revision Note
Gibbs Free Energy, ΔG
Gibbs free energy
As we have seen in the previous sections, the feasibility of a reaction is determined by two factors, the enthalpy change and the entropy change
The two factors come together in a fundamental thermodynamic concept called the Gibbs free energy (G)
The Gibbs equation is:
ΔGꝋ = ΔHreactionꝋ - TΔSsystemꝋ
The units of ΔGꝋare in kJ mol-1
The units of ΔHreactionꝋare in kJ mol-1
The units of T are in K
The units of ΔSsystemꝋare in J K-1 mol-1(and must therefore be converted to kJ K-1 mol-1 by dividing by 1000)
Calculating ΔGꝋ
There are two ways you can calculate the value of ΔGꝋ
From ΔHꝋand ΔSꝋ values
From ΔGꝋ values of all the substances present
Worked Example
Calculate the free energy change for the following reaction:
2NaHCO3 (s) → Na2CO3 (s) + H2O (l) + CO2 (g)
ΔHꝋ= +135 kJ mol-1 ΔS = +344 J K-1 mol-1
Answer
Step 1: Convert the entropy value in kilojoules
ΔSꝋ = +344 J K-1 mol-1 ÷ 1000 = +0.344 kJ K-1 mol-1
Step 2: Substitute the terms into the Gibbs Equation
ΔGꝋ = ΔHreactionꝋ - TΔSsystemꝋ
= +135 - (298 x 0.344)
= +32.49 kJ mol-1
The temperature is 298 K since standard values are quoted in the question
Worked Example
What is the standard free energy change, ΔGꝋ, for the following reaction?
C2H5OH(l) + 3O2(g) → 2CO2(g) + 3H2O(g)
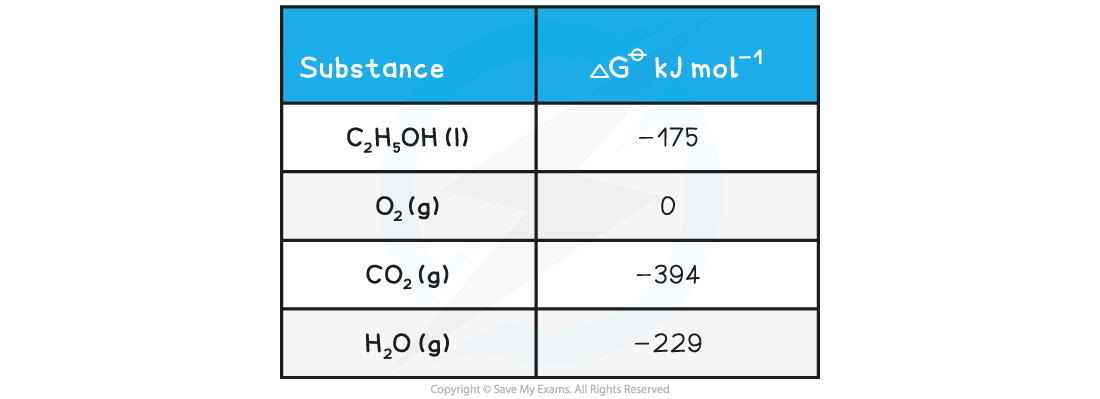
Answer
ΔGꝋ = ΣΔGproductsꝋ - ΣΔGreactantsꝋ
ΔGꝋ = [(2 x CO2 ) + (3 x H2O )] - [(C2H5OH) + (3 x O2)]
ΔGꝋ = [(2 x -394 ) + (3 x -229 )] - [-175 + 0]
ΔGꝋ = -1300 kJ mol-1
Free Energy & Equilibrium
Limitations of using ΔG
∆Go can only be used to predict the feasibility of a reaction under standard conditions
Under non-standard conditions, ∆G must be calculated
It is important to note that just because a reaction is feasible does not mean that it will occur at an observable rate
While ∆G can be used to determine the feasibility of a reaction, it does not take into account the kinetics of the reaction i.e. rate of reaction
There might be a large energy barrier (Ea) which the reacting species have to overcome before a reaction can occur
Some reactions are feasible since ∆G is negative, but kinetically not feasible since it just occurs too slowly
Such reactions are feasible but very slow
An example is the decomposition of hydrogen peroxide at 25 oC
H2O2 (l) → H2O (l) + ½O2 (g) ∆G = -117 kJ mol-1
This reaction has a very high Ea so must be catalysed using manganese dioxide, MnO2
If the reaction was left for long enough, the hydrogen peroxide would eventually decompose, however the addition of the MnO2 allows the reaction to take place via an alternative route with a lower Ea
Although the value for ∆G indicates the reaction is feasible, it does not take into account the kinetics of the reaction

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
