Calculations Using Born-Haber Cycles (OCR A Level Chemistry A) : Revision Note
Born-Haber Cycles - Calculations
Once a Born-Haber cycle has been constructed, it is possible to calculate the lattice energy (ΔHlattꝋ) by applying Hess’s law and rearranging:
ΔHfꝋ = ΔHatꝋ + ΔHatꝋ + IE + EA + ΔHlattꝋ
If we simplify this into three terms, this makes the equation easier to see:
ΔHlattꝋ
ΔHfꝋ
ΔH1ꝋ (the sum of all of the various enthalpy changes necessary to convert the elements in their standard states to gaseous ions)
The simplified equation becomes
ΔHfꝋ = ΔH1ꝋ + ΔHlattꝋ
So, if we rearrange to calculate the lattice energy, the equation becomes
ΔHlattꝋ = ΔHfꝋ - ΔH1ꝋ
When calculating the ΔHlattꝋ, all other necessary values will be given in the question
A Born-Haber cycle could be used to calculate any stage in the cycle
For example, you could be given the lattice energy and asked to calculate the enthalpy change of formation of the ionic compound
The principle would be exactly the same
Work out the direct and indirect route of the cycle (the stage that you are being asked to calculate will always be the direct route)
Write out the equation in terms of enthalpy changes and rearrange if necessary to calculate the required value
Remember: sometimes a value may need to be doubled or halved, depending on the ionic solid involved
For example, with MgCl2 the value for the first electron affinity of chlorine would need to be doubled in the calculation, because there are two moles of chlorine atoms
Therefore, you are adding 2 moles of electrons to 2 moles of chlorine atoms, to form 2 moles of Cl- ions
Worked Example
Given the data below, calculate the ΔHlattꝋ of potassium chloride (KCl)

Answer
Step 1: The corresponding Born-Haber cycle is:
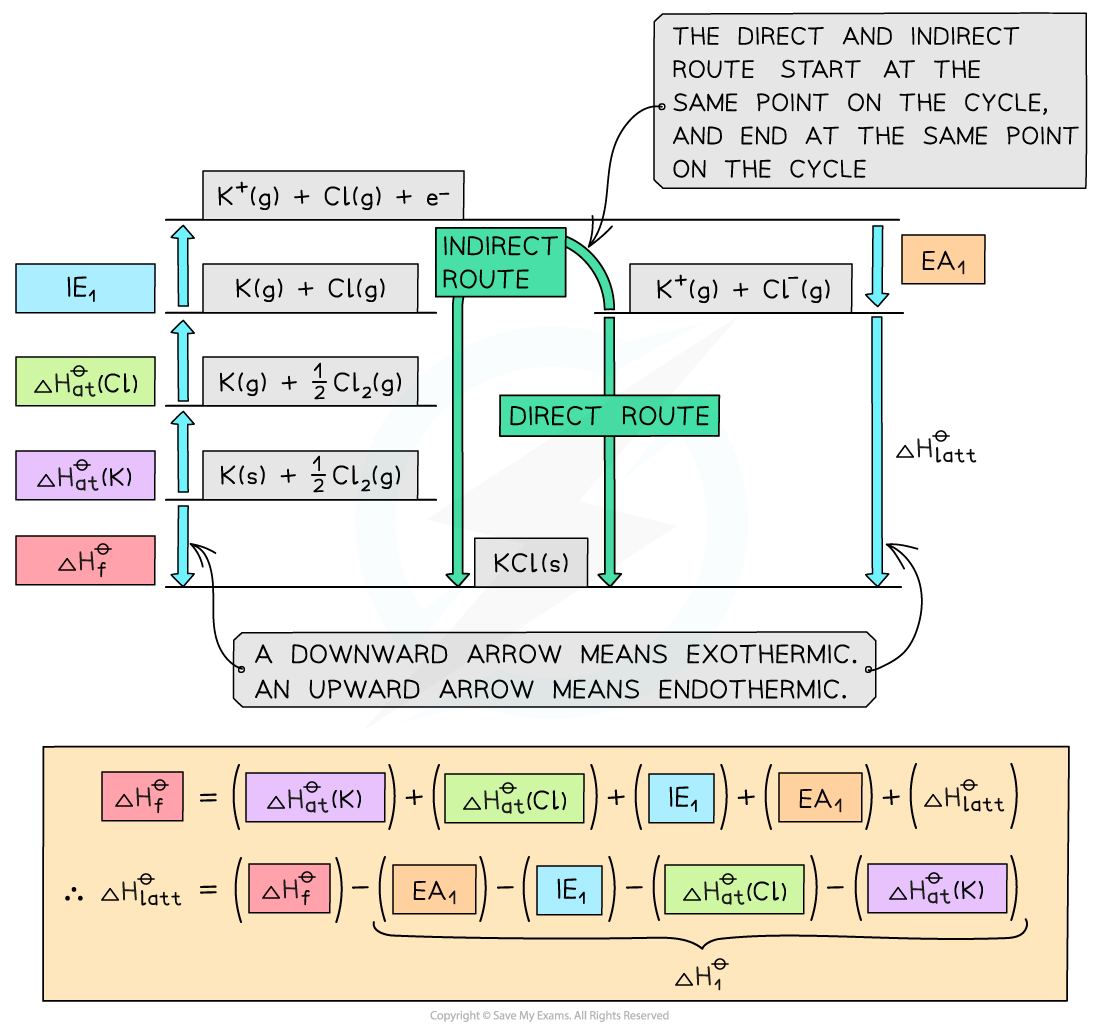
Step 2: Applying Hess’ law, the lattice energy of KCl is:
ΔHlattꝋ = ΔHfꝋ - ΔH1ꝋ
ΔHlattꝋ = ΔHfꝋ - [(ΔHat K) + (ΔHat Cl) + (IE1 K) + (EA1 Cl)]
Step 3: Substitute in the numbers:
ΔHlattꝋ = (-437) - [(+90) + (+122) + (+418) + (-349)] = -718 kJ mol-1
Worked Example
Given the data below, calculate the of ΔHlattꝋmagnesium oxide of magnesium oxide (MgO)

Answer
Step 1: The corresponding Born-Haber cycle is:
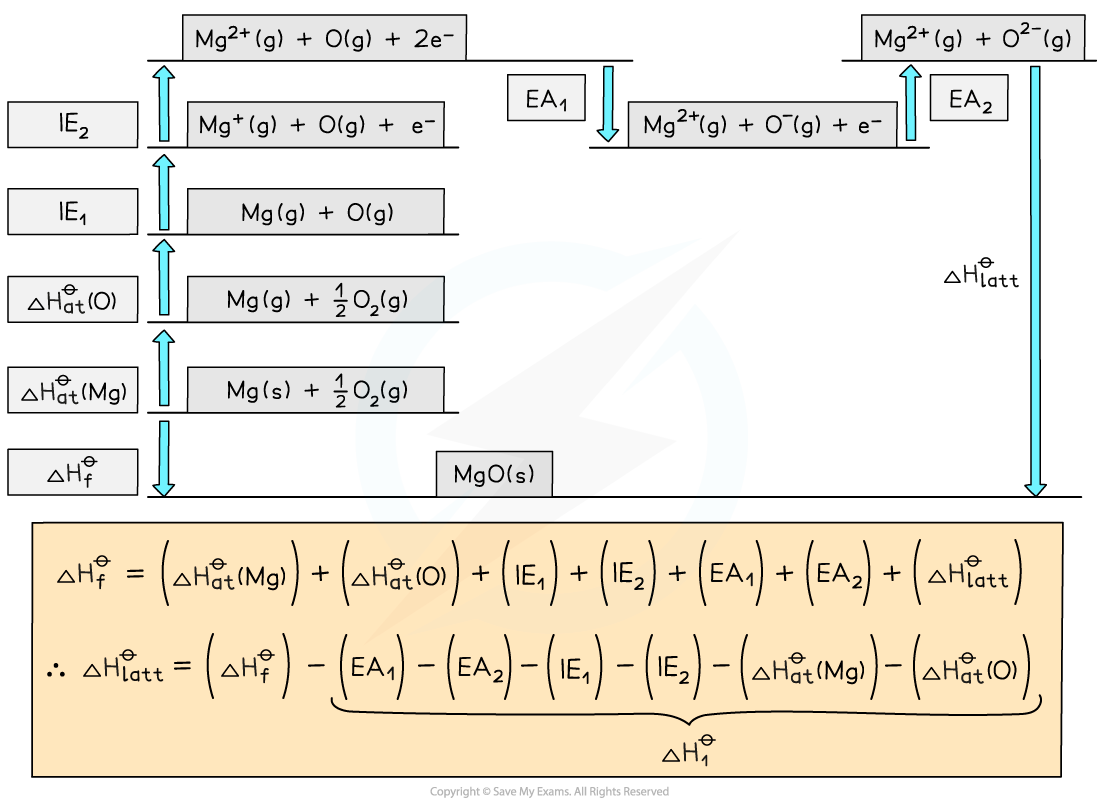
Step 2: Applying Hess’ law, the lattice energy of MgO is:
ΔHlattꝋ = ΔHfꝋ - ΔH1ꝋ
ΔHlattꝋ = ΔHfꝋ - [(ΔHatꝋ Mg) + (ΔHatꝋ O) + (IE1 Mg) + (IE2 Mg) + (EA1 O) + (EA2 O)]
Step 3: Substitute in the numbers:
ΔHlattꝋ = (-602) - [(+148) + (+248) + (+736) + (+1450) + (-142) + (+770)]
= -3812 kJ mol-1

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
