Buffers (OCR A Level Chemistry A) : Revision Note
Buffer Solutions
A buffer solution is a solution which resists changes in pH when small amounts of acids or alkalis are added
A buffer solution is used to keep the pH almost constant
A buffer can consists of weak acid - conjugate base or weak base - conjugate acid
Ethanoic acid & sodium ethanoate as a buffer
A common buffer solution is an aqueous mixture of ethanoic acid and sodium ethanoate
Ethanoic acid is a weak acid and partially ionises in solution to form a relatively low concentration of ethanoate ions

Sodium ethanoate is a salt which fully ionises in solution

There are reserve supplies of the acid (CH3COOH) and its conjugate base (CH3COO-)
The buffer solution contains relatively high concentrations of CH3COOH (due to partial ionisation of ethanoic acid) and CH3COO- (due to full ionisation of sodium ethanoate)
In the buffer solution, the ethanoic acid is in equilibrium with hydrogen and ethanoate ions

When H+ ions are added:
The equilibrium position shifts to the left as H+ ions react with CH3COO- ions to form more CH3COOH until equilibrium is re-established
As there is a large reserve supply of CH3COO- the concentration of CH3COO- in solution doesn’t change much as it reacts with the added H+ ions
As there is a large reserve supply of CH3COOH the concentration of CH3COOH in solution doesn’t change much as CH3COOH is formed from the reaction of CH3COO- with H+
As a result, the pH remains reasonably constant

When hydrogen ions are added to the solution the pH of the solution would decrease; However, the ethanoate ions in the buffer solution react with the hydrogen ions to prevent this and keep the pH constant
When OH- ions are added:
The OH- reacts with H+ to form water
OH- (aq) + H+ (aq) → H2O (l)
The H+ concentration decreases
The equilibrium position shifts to the right and more CH3COOH molecules ionise to form more H+and CH3COO- until equilibrium is re-established
CH3COOH (aq) → H+ (aq) + CH3COO- (aq)
As there is a large reserve supply of CH3COOH the concentration of CH3COOH in solution doesn’t change much when CH3COOH dissociates to form more H+ ions
As there is a large reserve supply of CH3COO- the concentration of CH3COO- in solution doesn’t change much
As a result, the pH remains reasonably constant
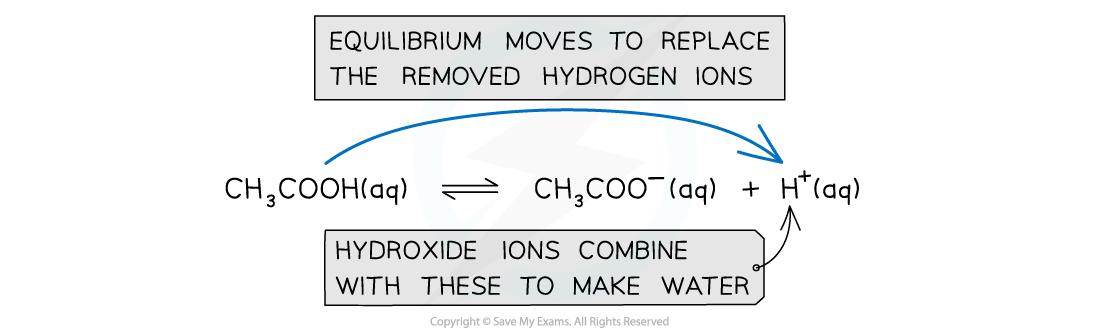
When hydroxide ions are added to the solution, the hydrogen ions react with them to form water; The decrease in hydrogen ions would mean that the pH would increase however the equilibrium moves to the right to replace the removed hydrogen ions and keep the pH constant
Calculating the pH of a Buffer Solution
The pH of a buffer solution can be calculated using:
The Ka of the weak acid
The equilibrium concentration of the weak acid and its conjugate base (salt)
To determine the pH, the concentration of hydrogen ions is needed which can be found using the equilibrium expression

To simplify the calculations, logarithms are used such that the expression becomes:

Since -log10 [H+] = pH, the expression can also be rewritten as:

This is known as the Hendersen-Hasselbalch equation
Worked Example
Calculate the pH of a buffer solution containing 0.305 mol dm-3 of ethanoic acid and 0.520 mol dm-3 sodium ethanoate.
The Ka of ethanoic acid = 1.74 × 10-5 mol dm-3 at 298 K
Answer
Ethanoic acid is a weak acid that ionises as follows:
CH3COOH (aq) ⇌ H+ (aq) + CH3COO- (aq)
Step 1: Write down the equilibrium expression to find Ka

Step 2: Rearrange the equation to find [H+]

Step 3: Substitute the values into the expression

= 1.02 x 10-5 mol dm-3
Step 4: Calculate the pH
pH = - log [H+]
= -log 1.02 x 10-5
= 4.99
Uses of Buffers
Controlling the pH of blood
In humans, HCO3- ions act as a buffer to keep the blood pH between 7.35 and 7.45
Body cells produce CO2 during aerobic respiration
This CO2 will combine with water in blood to form a solution containing H+ ions
CO2 (g) + H2O (l) ⇌ H+ (aq) + HCO3- (aq)
This equilibrium between CO2 and HCO3- is extremely important
If the concentration of H+ ions is not regulated, the blood pH would drop and cause ‘acidosis’
Acidosis refers to a condition in which there is too much acid in the body fluids such as blood
This could cause body malfunctioning and eventually lead to coma
If there is an increase in H+ ions
The equilibrium position shifts to the left until equilibrium is restored
CO2 (g) + H2O (l) ⇌ H+ (aq) + HCO3- (aq)
This reduces the concentration of H+ and keeps the pH of the blood constant
If there is a decrease in H+ ions
The equilibrium position shifts to the right until equilibrium is restored
CO2 (g) + H2O (l) ⇌ H+ (aq) + HCO3- (aq)
This increases the concentration of H+ and keeps the pH of the blood constant

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
