Ka, pH & Kw (OCR A Level Chemistry A) : Revision Note
The Acid Dissociation Constant, Ka
Weak acids
A weak acid is an acid that partially (or incompletely) dissociates in aqueous solutions
Eg. all organic acids e.g. ethanoic acid and some inorganic acids HCN (hydrocyanic acid), H2S (hydrogen sulfide) and H2CO3 (carbonic acid)
The position of the equilibrium is more over to the left and an equilibrium is established

The diagram shows the partial dissociation of a weak acid in aqueous solution
As this is an equilibrium we can write an equilibrium constant expression for the reaction

This constant is called the acid dissociation constant, Ka, and has the units mol dm-3
Values of Ka are very small, for example for ethanoic acid Ka = 1.74 x 10-5 mol dm-3
When writing the equilibrium expression for weak acids, the following assumptions are made:
The concentration of hydrogen ions due to the ionisation of water is negligible
The value of Ka indicates the extent of dissociation
The higher the value of Ka the more dissociated the acid and the stronger it is
The lower the value of Ka the weaker the acid
pKa
The range of values of Ka is very large and for weak acids, the values themselves are very small numbers
Table of Ka values
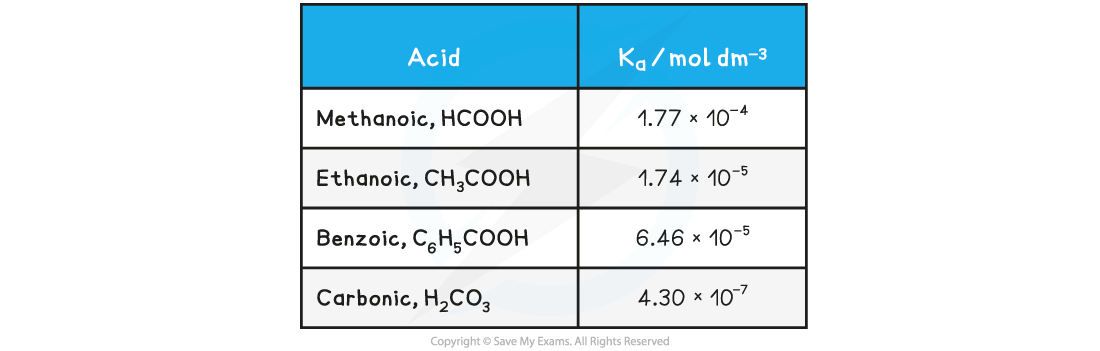
For this reason it is easier to work with another term called pKa
The pKa is the negative log of the Ka value, so the concept is analogous to converting [H+] into pH values
pKa = -logKa
Looking at the pKa values for the same acids:
Table of pKa values
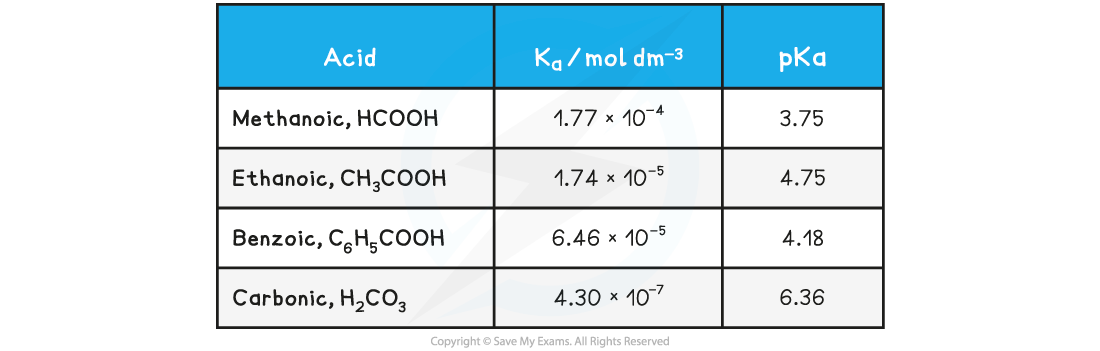
The range of pKa values for most weak acids lies between 3 and 7
pH & The Ionic Product of Water, Kw
pH
The acidity of an aqueous solution depends on the number of H+ (H3O+) ions in solution
The pH is defined as:
pH = -log[H+]
where [H+] is the concentration of hydrogen ions in mol dm–3
Similarly, the concentration of H+ of a solution can be calculated if the pH is known by rearranging the above equation to:
[H+] = 10-pH
The pH scale is a logarithmic scale with base 10
This means that each value is 10 times the value below it. For example, pH 5 is 10 times more acidic than pH 6.
pH values are usually given to 2 decimal places
The relationship between concentration is easily seen on the following table
pH & [H+] Table
![pH and [H+] Table, downloadable IB Chemistry revision notes](https://cdn.savemyexams.com/cdn-cgi/image/f=auto,width=3840/https://cdn.savemyexams.com/uploads/2021/06/8.1.6-pH-and-H-Table.png)
The ionic product of water, Kw
In all aqueous solutions, an equilibrium exists in water where a few water molecules dissociate into protons and hydroxide ions
We can derive an equilibrium constant for the reaction:

This is a specific equilibrium constant called the ionic product for water
The product of the two ion concentrations is always 1 x 10-14 mol2 dm-6
This makes it straightforward to see the relationship between the two concentrations and the nature of the solution:
[H+] & [OH–] Table
![[H+] and [OH-] table, downloadable IB Chemistry revision notes](https://cdn.savemyexams.com/cdn-cgi/image/f=auto,width=3840/https://cdn.savemyexams.com/uploads/2021/06/8.1.8-H-and-OH-table.png)

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
