The Biochemical Importance of Iron in Haemoglobin (OCR A Level Chemistry A): Revision Note
Exam code: H432
Ligand Substitution in Haemoglobin
Haemoglobin is one of nature's complexes using a transition metal ion
The haem molecule is a complex with iron(II) at its centre
Oxygen atoms form a dative covalent bond with the Fe(II) which enables oxygen molecules to be transported around the body in the blood

The haem molecule with iron(II) at its centre
Oxygen molecules are not very good ligands and bond weakly to the iron(II)
The weak bonds allows them to break off easily and be transported into cells
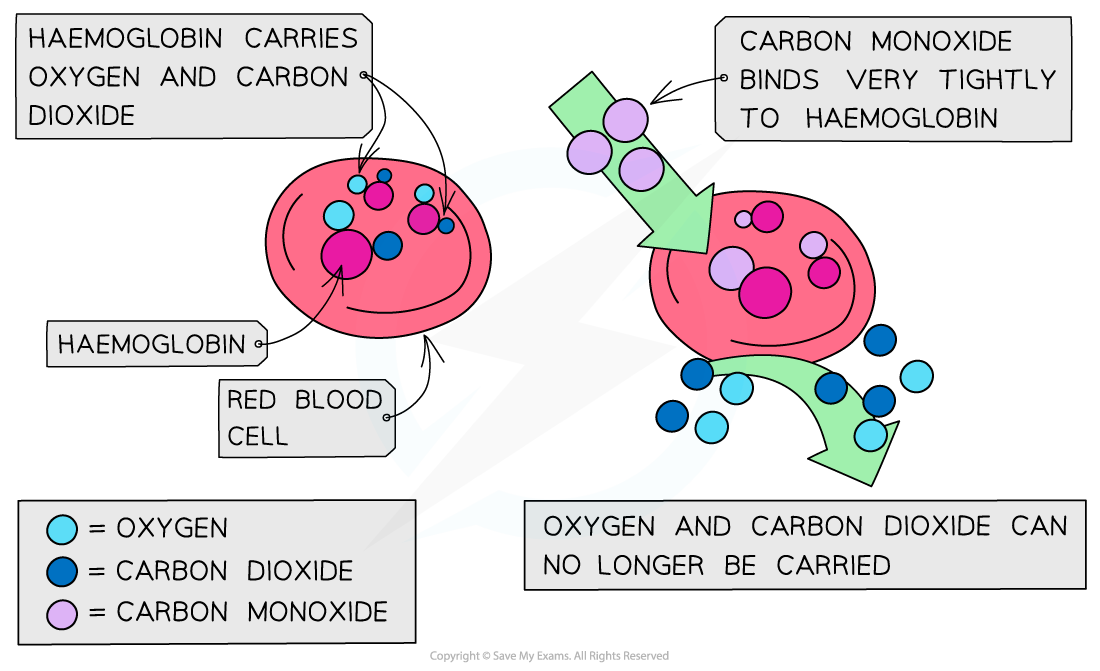
Carbon monoxide is toxic because it is a better ligand than oxygen and binds strongly and irreversibly to the iron(II) preventing oxygen from being carried to the cells
If oxygen attached to the haemoglobin (oxyhaemoglobin) is replaced by carbon monoxide (carboxyhaemoglobin), a darker red colour is produced in the haem complex
A sign of carbon monoxide poisoning
The condition anaemia occurs when a person does not have enough haemoglobin in their blood due to a loss of blood or deficiency in iron
Deficiency in iron can be restored by taking iron sulfate tablets in the diet

Unlock more, it's free!
Did this page help you?