The Feasibillity of a Reaction (OCR A Level Chemistry A): Revision Note
Exam code: H432
The Feasibility of a Reaction
The Gibbs equation can be used to calculate whether a reaction is feasible or not
ΔGꝋ = ΔHreactionꝋ - TΔSsystemꝋ
When ΔGꝋ is negative, the reaction is feasible and likely to occur
When ΔGꝋis positive, the reaction is not feasible and unlikely to occur
Feasible and spontaneous are fairly similar terms to describe reactions
Feasible tends to be used to describe reactions which are energetically favourable, so reactions that should go
Spontaneous tends to be used to describe reactions that go of their own accord
Worked Example
Calculate the Gibbs free energy change for the following reaction at 298 K and determine whether the reaction is feasible.
2Ca (s) + O2 (g) → 2CaO (s) ΔH = -635.5 kJ mol-1
Sꝋ[Ca(s)] = 41.00 J K-1 mol-1
Sꝋ[O2(g)] = 205.0 J K-1 mol-1
Sꝋ[CaO(s)] = 40.00 J K-1 mol-1
Answer
Step 1: Calculate ΔSsystemꝋ
ΔSsystemꝋ = ΣΔSproductsꝋ - ΣΔSreactantsꝋ
ΔSsystemꝋ = (2 x ΔSꝋ [CaO(s)]) - (2 x ΔSꝋ [Ca(s)] + ΔSꝋ [O2(g)])
= (2 x 40.00) - (2 x 41.00 + 205.0)
= -207.0 J K-1 mol-1
Step 2: Convert ΔSꝋ to kJ K-1 mol-1
= -207.0 J K-1 mol-1÷ 1000 = -0.207 kJ mol-1
Step 3: Calculate ΔGꝋ
ΔGꝋ = ΔHreactionꝋ - TΔSsystemꝋ
= -635.5 - (298 x -0.207)
= -573.8 kJ mol-1
Step 4: Determine whether the reaction is feasible
Since the ΔGꝋ is negative the reaction is feasible
Feasibility and Temperature
The feasibility of a reaction can be affected by the temperature
The Gibbs equation will be used to explain what will affect the feasibility of a reaction for exothermic and endothermic reactions

Exothermic reactions
In exothermic reactions, ΔHreactionꝋ is negative
If the ΔSsystemꝋ is positive:
Both the first and second term will be negative
Resulting in a negative ΔGꝋ so the reaction is feasible
Therefore, regardless of the temperature, an exothermic reaction with a positive ΔSsystemꝋ will always be feasible
If the ΔSsystemꝋ is negative:
The first term is negative and the second term is positive
At very high temperatures, the -TΔSsystemꝋ will be very large and positive and will overcome ΔHreactionꝋ
Therefore, at high temperatures ΔGꝋ is positive and the reaction is not feasible
Since the relative size of an entropy change is much smaller than an enthalpy change, it is unlikely that TΔS > ΔH as temperature increases
These reactions are therefore usually spontaneous at normal conditions
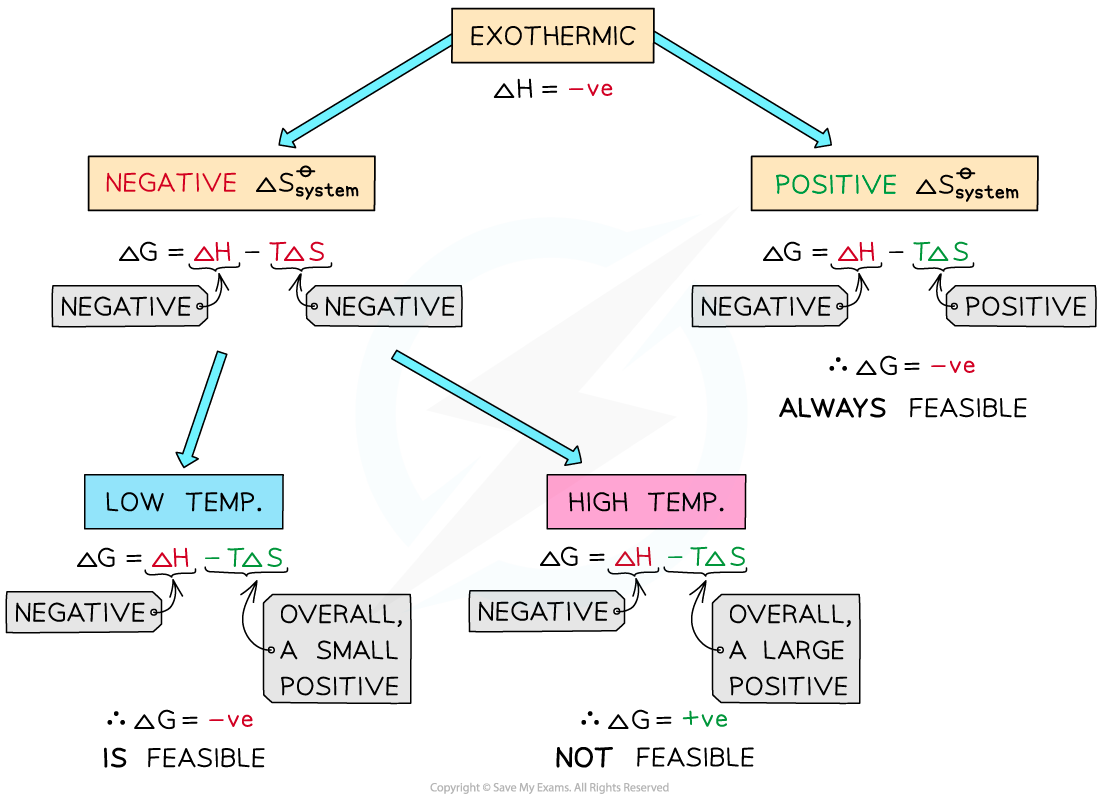
The diagram shows under which conditions exothermic reactions are feasible
Endothermic reactions
In endothermic reactions, ΔHreactionꝋ is positive
If the ΔSsystemꝋ is negative:
Both the first and second term will be positive
Resulting in a positive ΔGꝋ so the reaction is not feasible
Therefore, regardless of the temperature, endothermic with a negative ΔSsystemꝋ will never be feasible
If the ΔSsystemꝋ is positive:
The first term is positive and the second term is negative
At low temperatures, the -TΔSsystemꝋ will be small and negative and will not overcome the larger ΔHreactionꝋ
Therefore, at low temperatures ΔGꝋ is positive and the reaction is not feasible
The reaction is more feasible at high temperatures as the second term will become negative enough to overcome the ΔHreactionꝋ resulting in a negative ΔGꝋ
This tells us that for certain reactions which are not feasible at room temperature, they can become feasible at higher temperatures
An example of this is found in metal extractions, such as the extraction if iron in the blast furnace, which will be unsuccessful at low temperatures but can occur at higher temperatures (~1500 oC in the case of iron)
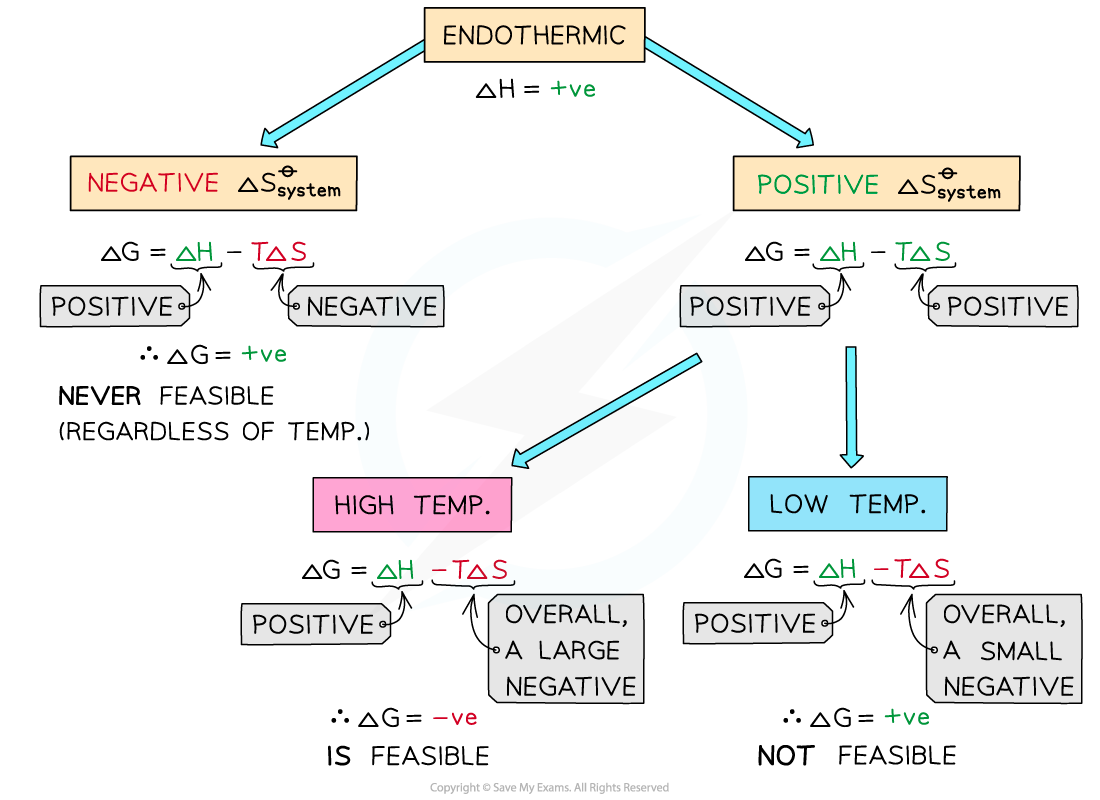
The diagram shows under which conditions endothermic reactions are feasible
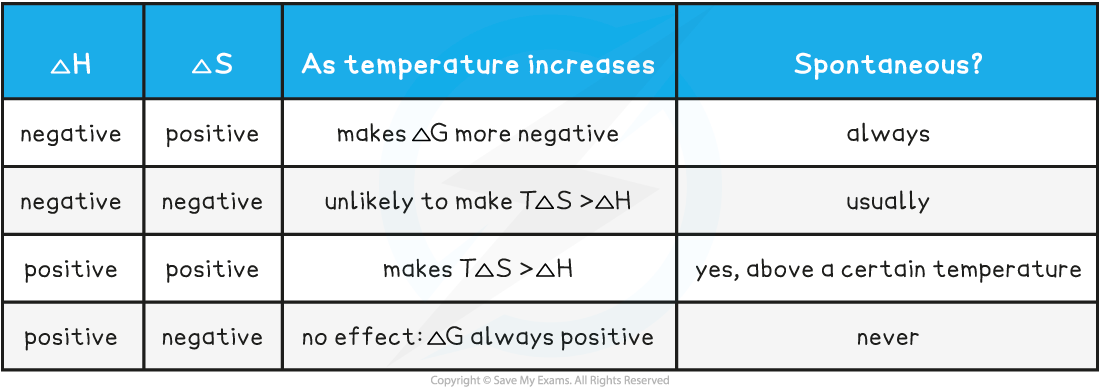
A summary table of free energy conditions

Unlock more, it's free!
Was this revision note helpful?
