Reactions of Alcohols (OCR A Level Chemistry A) : Revision Note
Combustion of Alcohols
Alcohols react with oxygen in the air when ignited and undergo complete combustion to form carbon dioxide and water
alcohol + oxygen → carbon dioxide + water

Complete combustion of alcohols to produce carbon dioxide and water
Lower alcohols burn with an almost invisible flame and make good fuels
Ethanol can be produced sustainably as a fuel by the fermentation of sugars
However, the energy density (the amount of energy in kJ per kg of fuel) is lower than gasoline so cars that run on ethanol must either have a larger fuel tank or fill up more often
Blending ethanol with gasoline or diesel increases the energy density and makes it safer in case of fires as it is easier to see the flames compared to pure ethanol burning
However, the are socio-economic concerns about using large quantities of farm land to produce crops for fermentation, which could be better used for food production
Oxidation of Alcohols
Primary alcohols can be oxidised to form aldehydes which can undergo further oxidation to form carboxylic acids
Secondary alcohols can be oxidised to form ketones only
Tertiary alcohols do not undergo oxidation
The oxidising agents of alcohols include acidified K2Cr2O7 or acidified KMnO4
Oxidising agents
The oxidising agents used to prepare aldehydes and ketones from alcohols include acidified potassium dichromate (K2Cr2O7) and acidified potassium manganate (KMnO4)
The acidified potassium dichromate(VI), K2Cr2O7, is an orange oxidising agent
When the alcohols are oxidised the orange dichromate ions (Cr2O72-) are reduced to green Cr3+ ions
The acidified potassium manganate(VII), KMnO4 is a purple oxidising agent
When the alcohols are oxidised the purple manganate ions (MnO4-) are reduced to colourless Mn2+ ions
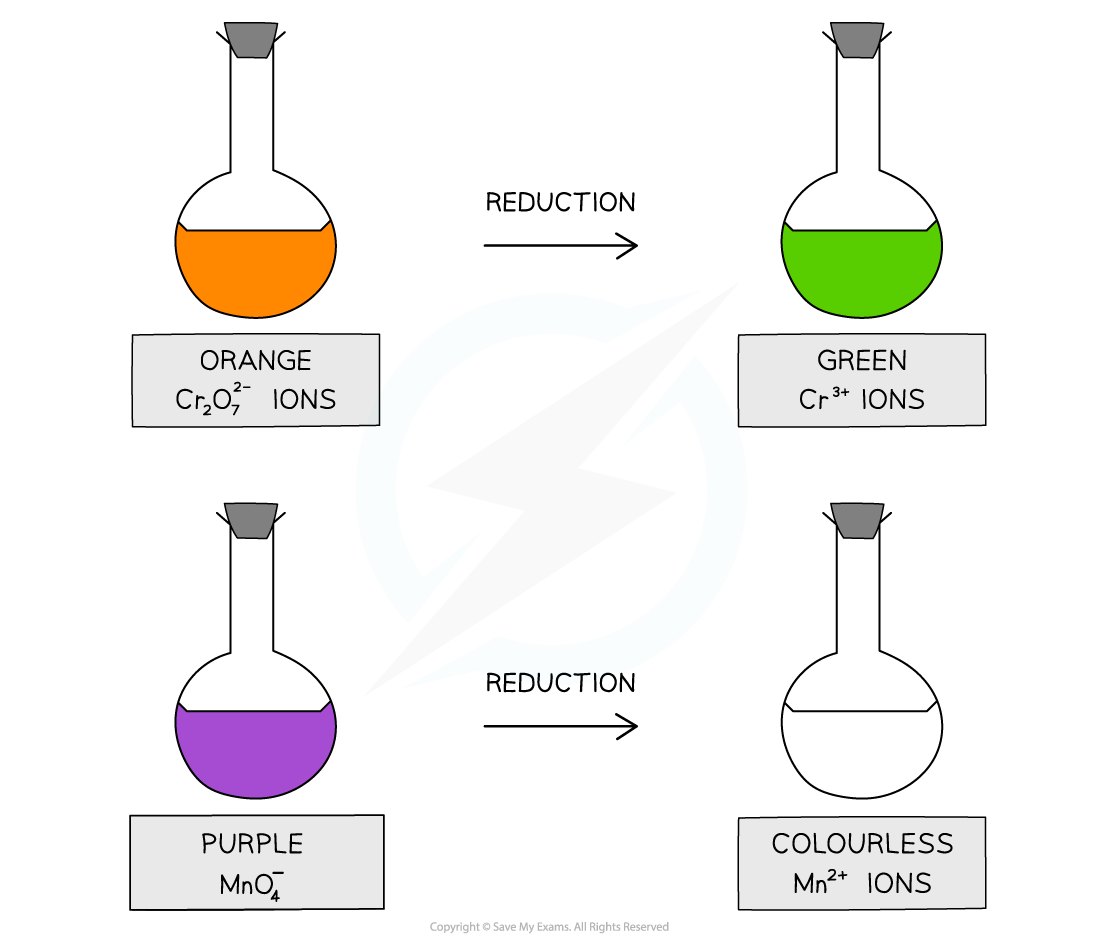
The oxidizing agents change colour when they oxidise an alcohol and get reduced themselves
Forming aldehydes and carboxylic acids
For aldehydes, a primary alcohol is added to the oxidising agent and warmed
The aldehyde product has a lower boiling point than the alcohol reactant so it can be distilled off as soon as it forms

Oxidation of ethanol by acidified K2Cr2O7 to form an aldehyde by distillation
If the aldehyde is not distilled off, further refluxing with excess oxidising agent will oxidise it to a carboxylic acid

Further oxidation of the aldehyde via reflux can be done to produce a carboxylic acid
Forming ketones

Oxidation of propan-2-ol by acidified K2Cr2O7 to form a ketone
Since ketones cannot be further oxidised, the ketone product does not need to be distilled off straight away after it has been formed
Elimination & Substitution Reactions of Alcohols
Elimination Reaction of Alcohols
Alcohols can also undergo dehydration to form alkenes
This is an example of an elimination reaction
Elimination reactions involve a small molecule leaving the parent molecule as a byproduct
In this case, the small molecule is a water molecule
The water molecule is formed from the -OH group and a hydrogen atom from the adjacent carbon atom
Alcohol vapour is passed over a hot catalyst of aluminium oxide (Al2O3) powder or pieces of porous pot
Excess hot, concentrated sulfuric acid or phosphoric acid is used as a catalyst

Dehydration of ethanol using aluminium oxide as a catalyst forms ethene gas, which can be collected over water
The reaction and mechanism for the dehydration of propan-2-ol is shown below

Dehydration of propan-2-ol mechanism
Substitution Reactions of Alcohols
In the substitution of alcohols, a hydroxy group (-OH) is replaced by a halogen to form an haloalkane
The substitution of the alcohol group for a halogen can be achieved by reacting the alcohol with:
HX (rather than using HBr, KBr is reacted with H2SO4 or H3PO4 to make HBr that will then react with the alcohol)

Substitution of alcohols to produce haloalkanes
Examiner Tips and Tricks
An alternative method to produce the bromoalkane by reacting the alcohol with sodium or potassium bromide and concentrated sulfuric acid generating HBr is to use phosphorus tribromide, PBr3.
To form bromoethane from ethanol, PBr3 can be added drop wise to ethanol (this reaction is very vigorous).
PBr3 + 3C2H5OH → 3C2H5Br + H3PO3
The water removes excess PBr3 by the hydrolysis reaction
PBr3 + 3H2O → 3HBr + H3PO3

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
