Coloured Ions (Edexcel A Level Chemistry): Revision Note
Exam code: 9CHO
Coloured Ions
Perception of colour
Most transition metal compounds appear coloured. This is because they absorb energy corresponding to certain parts of the visible electromagnetic spectrum
The colour that is seen is made up of the parts of the visible spectrum that aren’t absorbed
For example, a green compound will absorb all frequencies of the spectrum apart from green light, which is transmitted
The colours absorbed are complementary to the colour observed
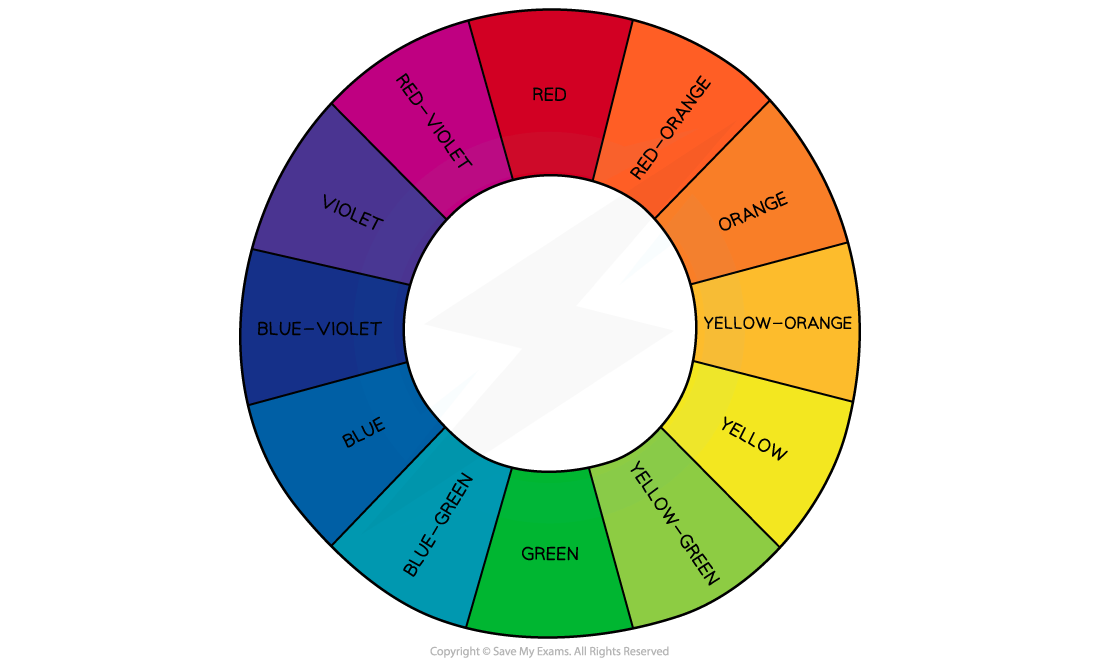
The colour wheel showing complementary colours in the visible light region of the electromagnetic spectrum
Complementary colours are any two colours which are directly opposite each other in the colour wheel
For example, the complementary colour of red is green and the complementary colours of red-violet are yellow-green
Splitting of 3d energy levels
In a transition metal atom, the five orbitals that make up the d-subshell all have the same energy.
Ions that have completely filled 3d energy levels (such as Zn2+) and ions that have no electrons in their 3d subshells (such as Sc3+) are not coloured
Transition metals have a partially filled 3d energy level
When ligands attach to the central metal ion the energy level splits into two levels with slightly different energies
If one of the electrons in the lower energy level absorbs energy from the visible spectrum it can move to the higher energy level
This process is known as promotion / excitation
The amount of energy absorbed depends on the difference between the energy levels
A larger energy difference means the electron absorbs more energy
The amount of energy gained by the electron is directly proportional to the frequency of the absorbed light and inversely proportional to the wavelength

Upon bonding to ligands, the d orbitals of the transition element ion split into sets of orbitals with different energies
Changes in Colour
The size of the splitting energy ΔE in the d-orbitals is influenced by the following four factors:
The size and type of ligands
The nuclear charge and identity of the metal ion
The oxidation state of the metal
The shape of the complex

The large variety of coloured compounds is a defining characteristic of transition metals
Size and type of ligand
The nature of the ligand influences the strength of the interaction between ligand and central metal ion
Ligands vary in their charge density
The greater the charge density; the more strongly the ligand interacts with the metal ion causing greater splitting of the d-orbitals
The greater the splitting, the more energy is needed to promote an electron to the higher group of orbitals
Therefore, the further it is shifted towards the region of the spectrum where it absorbs higher energy
As splitting increases, the light absorbed will shift away from the red end of the spectrum (longer wavelengths), towards the yellow end (shorter wavelengths)
As a result, a different colour of light is absorbed by the complex solution and a different complementary colour is observed
This means that complexes with the same transition elements ions, but different ligands, can have different colours
For example, the [Cu(H2O)6]2+ complex has a light blue colour
Whereas the [Cu(NH3)4(H2O)2]2+ has a dark blue colour despite the copper(II) ion having an oxidation state of +2 in both complexes

Ligand exchange of the water ligands by ammonia ligands causes a change in colour of the copper(II) complex solution
Oxidation number
When the same metal has a higher oxidation number that will also create a stronger interaction with the ligands
If you compare iron(II) and iron (III):
[Fe(H2O)6]2+ absorbs in the red region and appears green
But, [Fe(H2O)6]3+ absorbs in blue region and appears orange
Coordination number
The change of colour in a complex is also partly due to the change in coordination number and geometry of the complex ion
The splitting energy, ΔE, of the d-orbitals is affected by the relative orientation of the ligand as well as the d-orbitals
Changing the coordination number generally involves changing the ligand as well, so it is a combination of these factors that alters the strength of the interactions

Unlock more, it's free!
Did this page help you?