Redox Titration Calculations (Edexcel A Level Chemistry): Revision Note
Exam code: 9CHO
Redox Titration Calculations
Redox Titrations
In a titration, the concentration of a solution is determined by titrating with a solution of known concentration.
In redox titrations, an oxidising agent is titrated against a reducing agent
Electrons are transferred from one species to the other
Indicators are sometimes used to show the endpoint of the titration
However, most transition metal ions naturally change colour when changing oxidation state
There are two common redox titrations you should know about manganate(VII) titrations and iodine-thiosulfate titrations
Potassium manganate(VII) titrations
In these redox titrations the manganate(VII) is the oxidising agent and is reduced to Mn2+(aq)
The iron is the reducing agent and is oxidised to Fe3+(aq) and the reaction mixture must be acidified, to excess acid is added to the iron(II) ions before the reaction begins
The choice of acid is important, as it must not react with the manganate(VII) ions, so the acid normally used is dilute sulfuric acid
As it does not oxidise under these conditions and does not react with the manganate(VII) ions
You could be asked why other acids are not suitable for this redox titration in the exam so make sure you understand the suitability of dilute sulfuric acid
Table explaining why other acids are not suitable for the redox titration

Indicator and end point
Potassium manganate(VII) titrations are self-indicating
Potassium manganate(VII) solution is purple
The potassium manganate(VII) solution is titrated into the conical flask from the burette
The manganate(VII) ions react with the analyte to form manganese(II) ions
The manganese(II) ions, Mn2+(aq), have a very pale pink colour
But, the concentration is so low that the solution looks colourless
When all of the analyte ions have reacted with the manganate(VII) ions, a pale pink colour appears in the flask due to an excess of manganate(VII) ions
When this colour remains with swirling, the endpoint has been reached
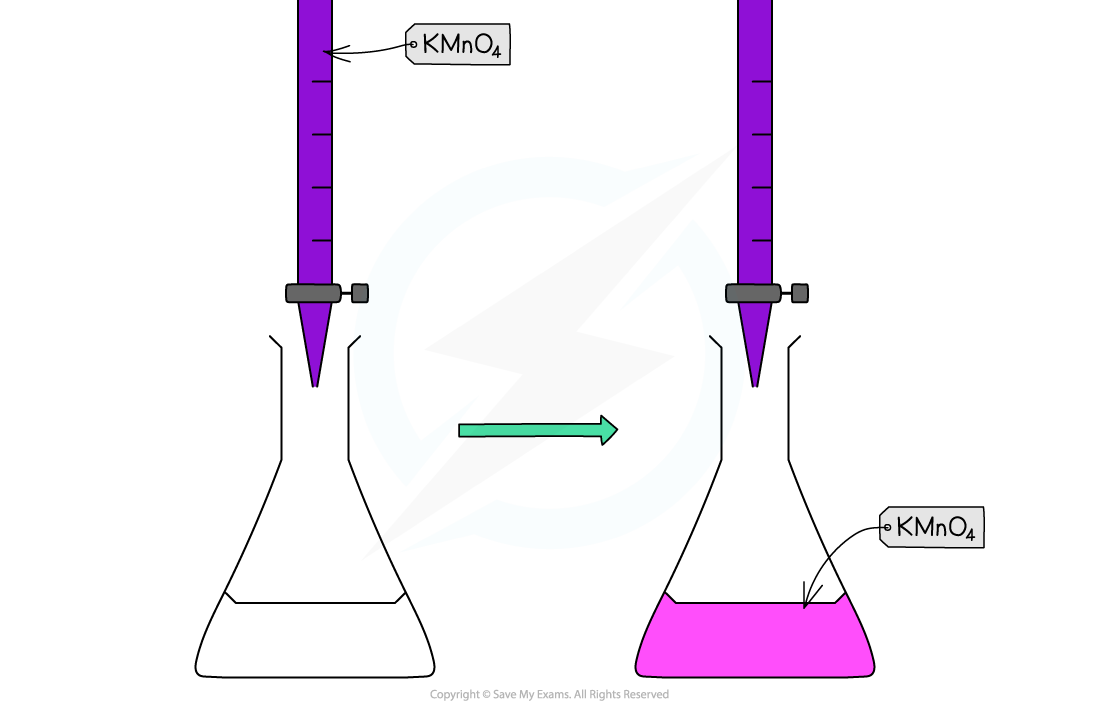
Redox titration colour change for potassium permanganate and iron(II) ions
Worked Example
Equations
Find the stoichiometry for the reaction and complete the two half equations:
MnO4- (aq) + 5e- + 8H+ (aq) → Mn2+ (aq) + 4H2O (l)
Fe2+ (aq) → Fe3+ (aq) + e-
Answers:
Balance the electrons:
MnO4- (aq) + 5e- + 8H+ (aq) → Mn2+ (aq) + 4H2O (l)
5Fe2+ (aq) → 5Fe3+ (aq) + 5e-
Add the two half equations:
MnO4- (aq) + 8H+ (aq) 5Fe2+ (aq) → Mn2+ (aq) + 4H2O (l) + 5Fe3+ (aq)
Manganate(VII) titrations can be used to determine:
The percentage purity of iron supplements
Percentage purity =
The formula of a sample of hydrated ethanedioic acid
Worked Example
Analysis of iron tablets
An iron tablet, weighing 0.960 g was dissolved in dilute sulfuric acid. An average titre of 28.50 cm3 of 0.0180 mol dm-3 potassium manganate(VII) solution was needed to reach the endpoint.
What is the percentage by mass of iron in the tablet?
Answer:
MnO4- (aq) + 8H+ (aq) + 5Fe2+ → Mn2+ (aq) + 5Fe3+ (aq) + 4H2O (l)
1 : 5 ratio of MnO4- : Fe2+
Number of moles of MnO4- (aq)
5.13 x 10-4 moles
Moles of iron(II) = 5 x 5.13 x 10-4 = 2.565 x 10-3 moles
Mass of iron(II) = 55.8 x 2.565 x 10-3 = 0.143127 g
Percentage by mass =
14.9%
Iodine-Thiosulfate Titrations
A redox reaction occurs between iodine and thiosulfate ions:
2S2O32– (aq) + I2 (aq) → 2I–(aq) + S4O62– (aq)
The light brown/yellow colour of the iodine turns paler as it is converted to colourless iodide ions
When the solution is a straw colour, starch is added to clarify the end point
The solution turns blue/black until all the iodine reacts, at which point the colour disappears.
This titration can be used to determine the concentration of an oxidizing agent, which oxidizes iodide ions to iodine molecules
The amount of iodine is determined from titration against a known quantity of sodium thiosulfate solution
Worked Example
Analysis of household bleach
Chlorate(I) ions, ClO-, are the active ingredient in many household bleaches.
10.0 cm3 of bleach was made up to 250.0 cm3. 25.0 cm3 of this solution had 10.0 cm3 of 1.0 mol dm-3 potassium iodide and then acidified with 1.0 mol dm-3 hydrochloric acid.
ClO- (aq) + 2I- (aq) + 2H+ (aq) → Cl- (aq) + I2 (aq) + H2O (l)
This was titrated with 0.05 mol dm-3 sodium thiosulfate solution giving an average titre of 25.20 cm3.
2S2O32- (aq) + I2 (aq) → 2I- (aq) + S4O62- (aq)
What is the concentration of chlorate(I) ions in the bleach?
Answer:
One mole of ClO- (aq) produces one mole of I2 (aq) which reacts with two moles of 2S2O32- (aq)
Therefore, 1 : 2 ratio of ClO- (aq) : S2O32- (aq)
Number of moles of S2O32- (aq)
1.26 x 10-3 moles
Number of moles of I2 (aq) and ClO- (aq) in 25.0 cm3
6.30 x 10-4 moles
Number of moles of ClO- (aq) in 250.0 cm3 = 6.30 x 10-4 x 10 = 6.30 x 10-3 moles
The 250.0 cm3 was prepared from 10.0 cm3 bleach
10 cm3 bleach = 6.30 x 10-3 moles of ClO- ions
1.0 dm3 bleach = 0.630 moles of ClO- ions
Therefore, the concentration of ClO- ions in the bleach is 0.630 mol dm-3
Examiner Tips and Tricks
General sequence for redox titration calculations
Write down the half equations for the oxidant and reductant
Deduce the overall equation
Calculate the number of moles of manganate(VII) or dichromate(VI) used
Calculate the ratio of moles of oxidant to moles of reductant from the overall redox equation
Calculate the number of moles in the sample solution of the reductant
Calculate the number of moles in the original solution of reductant
Determine either the concentration of the original solution or the percentage of reductant in a known quantity of sample

Unlock more, it's free!
Did this page help you?