Polarising Power (Edexcel A Level Chemistry): Revision Note
Exam code: 9CHO
Polarisation of Ions
Theoretical lattice energies assume a perfect ionic model where the ions are 100% spherical and the attractions are purely electrostatic
So overall, there has a been a complete and irreversible exchange of outer shell electrons between the two elements in the compound so that one ion is negatively charged and the other ion positively charged and the charges are whole values
Theoretical lattice energy can differ from measured lattice energy
This is because covalent character is introduced when there is polarisation of the anions
When this occurs, the cation attracts electrons from the anion therefore distorting electron density of the anion
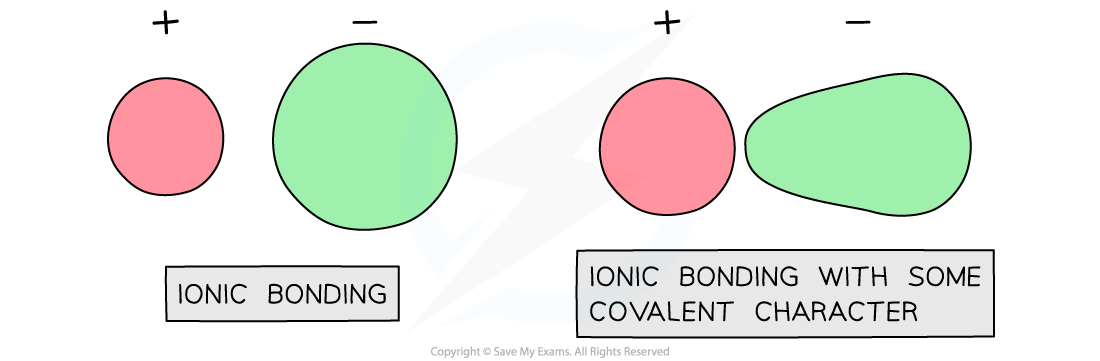
Polarisation of anion leading to covalent character
Anions & Cations
Cations
A cation with a large charge density will have a larger polarising power
The charge density can be calculated as the charge of the cation divided by the surface area, for example
K+ will have a lower polarising power than Li+ as the ionic radius is greater
Na+ will have a lower polarising power than Mg2+ as the charge is smaller
So a cation that is small and highly charged will have the greatest polarising power
Anions
Anions are polarised
The polarisability of the anion depends of its ionic radius
The larger the ionic radius the more easily it will be distorted, for example
Br- will be more polarisable than Cl- as the ionic radius is larger

Unlock more, it's free!
Did this page help you?