Ionic Product of Water (Edexcel A Level Chemistry): Revision Note
Exam code: 9CHO
Ionic Product of Water, Kw
In all aqueous solutions, an equilibrium exists in water where a few water molecules dissociate into protons and hydroxide ions
We can derive an equilibrium constant for the reaction:

This is a specific equilibrium constant called the ionic product for water
The product of the two ion concentrations is always 1 x 10-14 mol2 dm-6
This makes it straightforward to see the relationship between the two concentrations and the nature of the solution:
[H+] & [OH–] Table
![[H+] and [OH-] table, downloadable IB Chemistry revision notes](https://cdn.savemyexams.com/cdn-cgi/image/f=auto,width=3840/https://cdn.savemyexams.com/uploads/2021/06/8.1.8-H-and-OH-table.png)
The relationship between Kw and pKw is given by the following equation:
pKw = -logKw
pKa
The range of values of Ka is very large and for weak acids, the values themselves are very small numbers
Table of Ka values
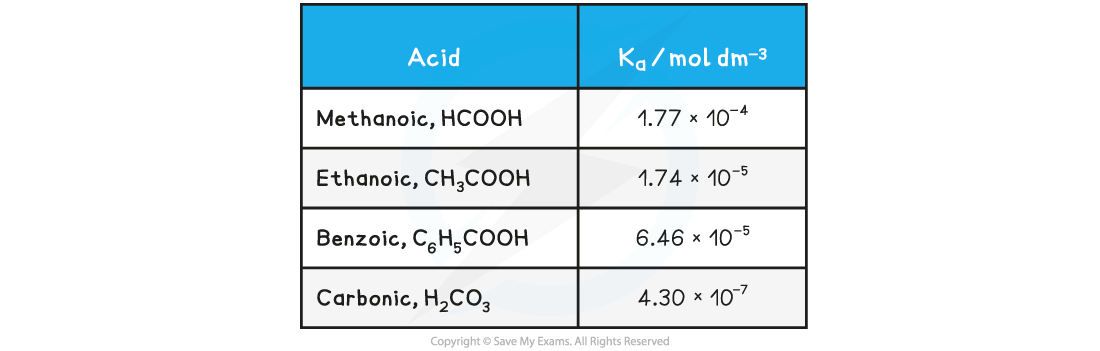
For this reason it is easier to work with another term called pKa
The pKa is the negative log of the Ka value, so the concept is analogous to converting [H+] into pH values
pKa = -logKa
Looking at the pKa values for the same acids:
Table of pKa values
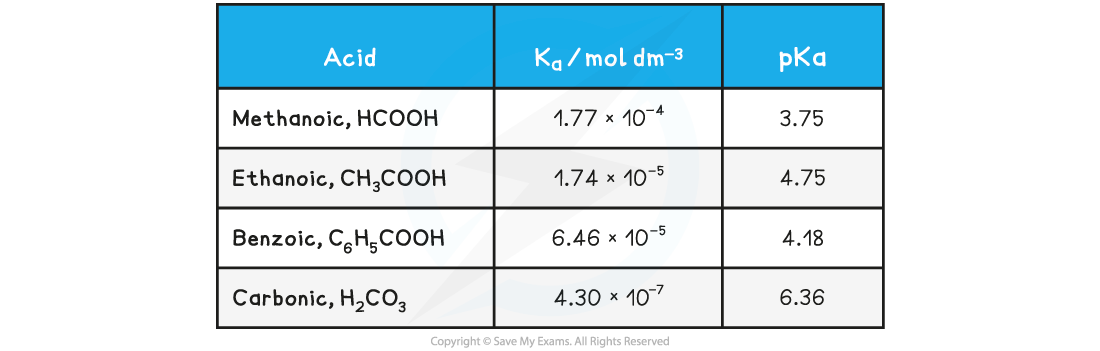
The range of pKa values for most weak acids lies between 3 and 7
pH Calculation of a Strong Base
Strong bases are completely ionised in solution
BOH (aq) → B+ (aq) + OH- (aq)
Therefore, the concentration of hydroxide ions [OH-] is equal to the concentration of base [BOH]
Even strong alkalis have small amounts of H+ in solution which is due to the ionisation of water
The concentration of OH- in solution can be used to calculate the pH using the ionic product of water
Once the [H+] has been determined, the pH of the strong alkali can be founding using pH = -log[H+]

Similarly, the ionic product of water can be used to find the concentration of OH- ions in solution if [H+] is known, simply by dividing Kw by the [H+]
Worked Example
Sodium hydroxide is a strong base which ionises as follows:
NaOH (aq) → Na+ (aq) + OH- (aq)
Calculate the pH of 0.15 mol dm-3 sodium hydroxide, NaOH
Answer 1:
The pH of the solution is:
[H+] =
[H+] = = 6.66 x 10-14
pH = -log[H+]
= -log 6.66 x 10-14 = 13.17
Worked Example
Sodium hydroxide is a strong base which ionises as follows:
NaOH (aq) → Na+ (aq) + OH- (aq)
Calculate the hydroxide concentration of a solution of sodium hydroxide when the pH is 10.50
Step 1: Calculate hydrogen concentration by rearranging the equation for pH
pH = -log[H+]
[H+]= 10-pH
[H+]= 10-10.50
[H+]= 3.16 x 10-11 mol dm-3
Step 2: Rearrange the ionic product of water to find the concentration of hydroxide ions
Kw = [H+] [OH-]
[OH-] =
Step 3: Substitute the values into the expression to find the concentration of hydroxide ions
Since Kw is 1 x 10-14 mol2 dm-6
[OH-]=
[OH-]= 3.16 x 10-4 mol dm-3

Unlock more, it's free!
Was this revision note helpful?