Period 3 Chlorides (Cambridge (CIE) A Level Chemistry): Revision Note
Exam code: 9701
Reaction of Period 3 Chlorides & Water
Chlorides of Period 3 elements show characteristic behaviour when added to water which can be explained by looking at their chemical bonding and structure
Chemical bonding & structure of Period 3 chlorides table
Period 3 chloride | NaCl | MgCl2 | Al2Cl6 | SiCl4 | PCl5 | SCl2 |
|---|---|---|---|---|---|---|
Chemical bonding | Ionic | Ionic | Covalent | Covalent | Covalent | Covalent |
Structure | Giant ionic | Giant ionic | Simple molecular | Simple molecular | Simple molecular | Simple molecular |
Observations | White solids dissolve to form colourless solutions | Chlorides react with water giving off white fumes of hydrogen chloride gas | ||||
pH of solution formed | 7.0 | 6.5 | 3.0 | 2.0 | 2.0 | 2.0 |
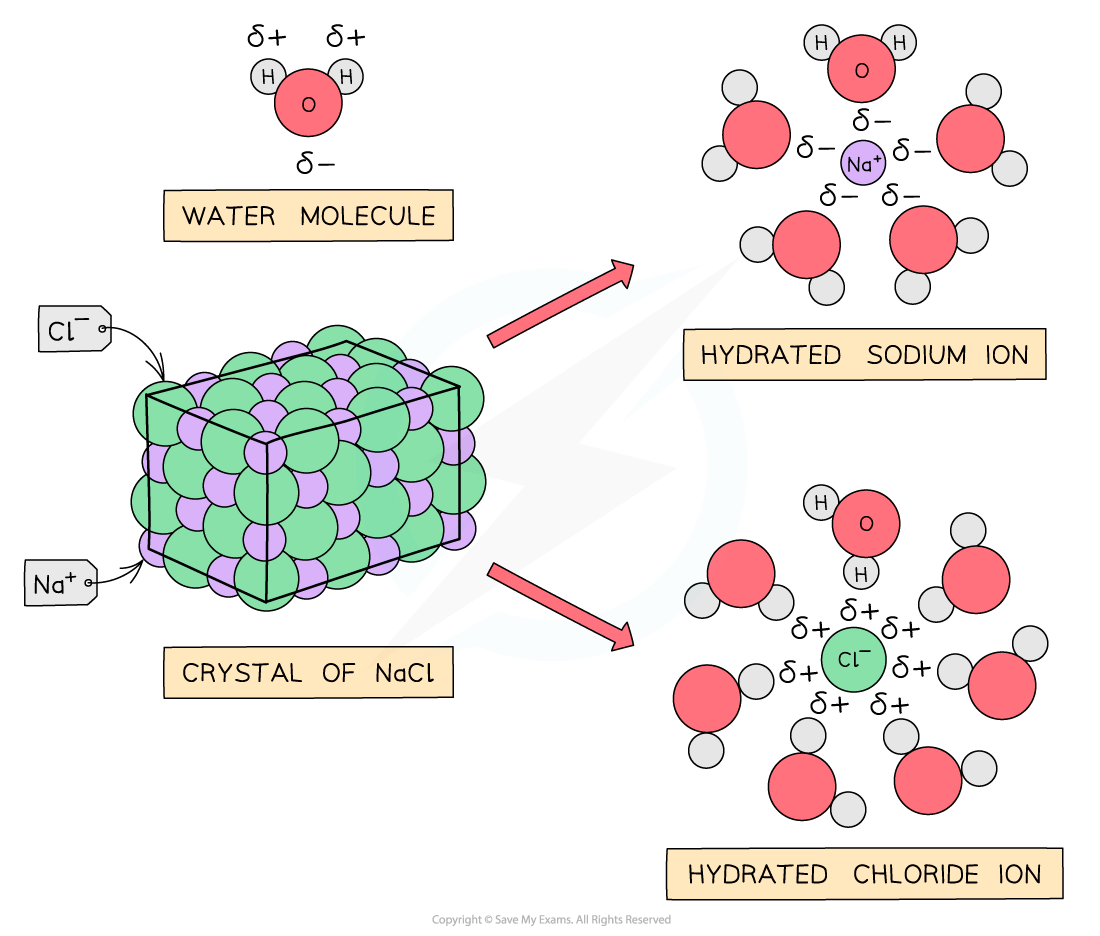
Sodium & magnesium chloride
NaCl and MgCl2 do not react with water as the polar water molecules are attracted to the ions dissolving the chlorides and breaking down the giant ionic structures: the metal and chloride ions become hydrated ions
Aluminium chloride
Aluminium chloride exists in two different forms depending on conditions:
Anhydrous AlCl3 is covalent and exists as a dimer, Al2Cl6
In aqueous solution, AlCl3 dissociates into Al3+ and Cl- ions and behaves ionically
The two forms of aluminium chloride

When water is added to aluminium chloride the dimers are broken down and Al3+ and Cl- ions enter the solution
The highly charged Al3+ ion becomes hydrated and causes a water molecule that is bonded to the Al3+ to lose an H+ ion which turns the solution acidic
The H+ and the Cl- form hydrogen chloride gas which is given off as white fumes
How the Al3+ makes an acidic solution

Silicon chloride
SiCl4 is hydrolysed in water, releasing white fumes of hydrogen chloride gas in a rapid reaction
SiCl4 (l) + 2H2O (l) → SiO2 (s) + 4HCl (g)
The SiO2 is seen as a white precipitate and some of the hydrogen chloride gas produced dissolves in water to form an acidic solution
Phosphorus(V) chloride
PCl5 also gets hydrolysed in water
PCl5 (s) + 4H2O (l) → H3PO4 (aq) + 5HCl (g)
Both H3PO4 and dissolved HCl are highly acidic

Unlock more, it's free!
Did this page help you?