Reactions of Arenes (Cambridge (CIE) A Level Chemistry): Revision Note
Exam code: 9701
Reactions of Arenes
Arenes are very stable compounds due to the delocalisation of π electrons in the ring
This is because the negative charge is spread out over the molecule instead of being confined to a small area
During chemical reactions such as substitution reactions, this delocalised ring is maintained
Addition reactions however, disrupt the aromatic stabilisation
Benzene undergoes a wide range of reactions including combustion - (complete and incomplete) and the following reactions:
Halogenation
Nitration
Friedel-Craft's alkylation
Friedel-Craft's acylation
Complete Oxidation
Hydrogenation
Halogenation
Halogenation reactions are examples of electrophilic substitution reactions
Arenes undergo substitution reactions with chlorine (Cl2) and bromine (Br2) in the presence of anhydrous AlCl3 or AlBr3 catalyst respectively to form halogenoarenes (aryl halides)
The chlorine or bromine acts as an electrophile and replaces a hydrogen atom on the benzene ring
The catalyst is required for the reaction to take place, due to the stability of the benzene structure
Halogenation of benzene

Arenes undergo substitution reactions with halogens to form aryl halides
Alkylarenes such as methylbenzene undergo halogenation on the 2 or 4 positions
This is due to the electron-donating alkyl groups which activate these positions
Phenol (C6H5OH) and phenylamine (C6H5NH2) are also activated in the 2 and 4 positions
The halogenation of alkylarenes, therefore, results in the formation of two products
Halogenation of alkylarenes

Alkylarenes are substituted on the 2 or 4 position
Multiple substitutions occur when excess halogen is used
Halogenation of alkylarenes using an excess of halogen

In the presence of excess halogen, multiple substitutions occur
Nitration
Another example of a substitution reaction is the nitration of arenes
In these reactions, a nitro (-NO2) group replaces a hydrogen atom on the arene
The benzene is reacted with a mixture of concentrated nitric acid (HNO3) and concentrated sulfuric acid (H2SO4) at a temperature between 25 and 60 oC
Nitration of benzene

During nitration, a hydrogen atom is replaced by an NO2 group
Again, due to the electron-donating alkyl groups in alkylarenes, nitration of methylbenzene will occur on the 2 and 4 position
Nitration of alkylarenes
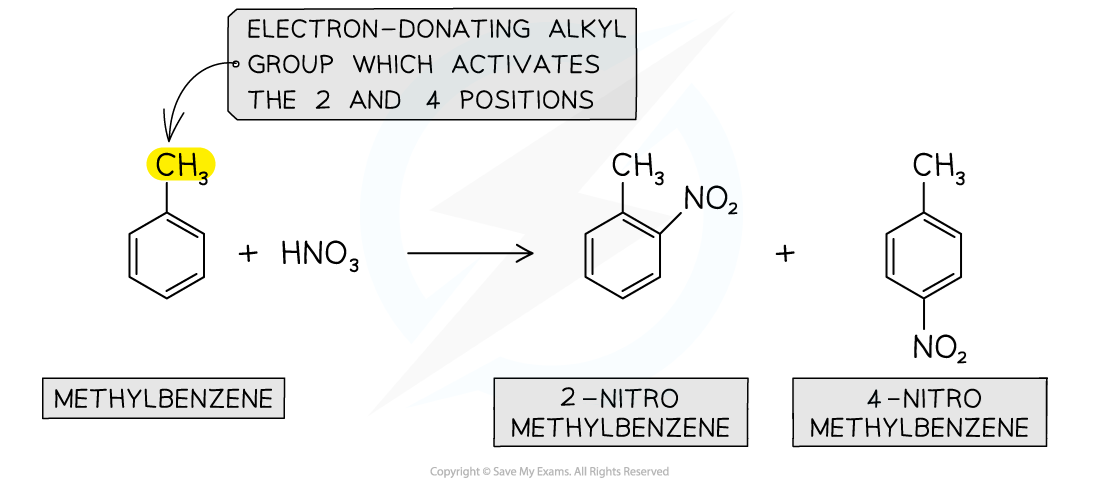
Alkylarenes are nitrated on the 2 or 4 position
Friedel-Crafts reactions
Friedel-Crafts reactions are also electrophilic substitution reactions
Due to the aromatic stabilisation in arenes, they are often unreactive
To use arenes as starting materials for the synthesis of other organic compounds, their structure, therefore, needs to be changed to turn them into more reactive compounds
Friedel-Crafts reactions can be used to substitute a hydrogen atom in the benzene ring for an alkyl group (Friedel-Crafts alkylation) or an acyl group (Friedel-Crafts acylation)
Like any other electrophilic substitution reaction, the Friedel-Crafts reactions consist of three steps:
Generating the electrophile
Electrophilic attack on the benzene ring
Regenerating aromaticity of the benzene ring
Examples of Friedel-Crafts alkylation and acylation reactions

During alkylation, an alkyl / R group is substituted on the benzene ring and during acylation, an acyl / RCO group is substituted on the benzene ring
Friedel-Crafts alkylation
In this type of Friedel-Crafts reaction, an alkyl chain is substituted into the benzene ring
The benzene ring is reacted with a chloroalkane in the presence of an AlCl3 catalyst
An example of an alkylation reaction is the reaction of benzene with chloropropane (CH3CH2CH2Cl) to form propylbenzene
Example of a Friedel-Crafts alkylation reaction


Alkylation reactions of benzene follow the 3 steps of electrophile generation, electrophilic attack and regeneration of aromaticity
Friedel-Crafts acylation
In the Friedel-Crafts acylation reaction, an acyl group is substituted into the benzene ring
An acyl group is an alkyl group containing a carbonyl, C=O group
The benzene ring is reacted with an acyl chloride in the presence of an AlCl3 catalyst
An example of an acylation reaction is the reaction of methylbenzene with propanoyl chloride to form an acyl benzene
Note that the acyl group substitutes on the 4 position due to the -CH3 group on the benzene
Example of a Friedel-Crafts acylation reaction


Acylation reactions of benzene follow the same 3 steps of electrophile generation, electrophilic attack and regeneration of aromaticity
Complete oxidation
Normally, alkanes are not oxidised by oxidising agents such as potassium manganate(VII) (KMnO4)
However, the presence of the benzene ring in alkyl arenes affects the properties of the alkyl side-chain
The alkyl side-chains in alkyl arenes are oxidised to carboxylic acids when refluxed with alkaline potassium manganate(VII) and then acidified with dilute sulfuric acid (H2SO4)
For example, the complete oxidation of ethylbenzene forms benzoic acid
Oxidation of alkylarenes

The complete oxidation of alkyl side chains in arenes gives a carboxylic acid
Hydrogenation
The hydrogenation of benzene is an addition reaction
Benzene is heated with hydrogen gas and a nickel or platinum catalyst to form cyclohexane
Hydrogenation of benzene

Hydrogenation of benzene results in a loss of aromaticity
The same reaction occurs when ethylbenzene undergoes hydrogenation to form cycloethylbenzene
Hydrogenation of methylbenzene

Hydrogenation of alkylarenes also results in a loss of aromaticity
Summary of Reactions of Arenes Table
Reaction | Conditions | Products |
|---|---|---|
Halogenation | Cl2 with an AlCl3 catalyst Br2 with an AlBr3 catalyst | Aryl halide |
Nitration | A mixture of concentrated H2SO4 and concentrated HNO3 Temperature between 25 oC and 60 oC | Nitroarene |
Friedel-Crafts alkylation | Halogenoalkane and anhydrous AlCl3 catalyst | Alkylbenzene |
Freidel-Crafts acylation | Acyl chloride and anhydrous AlCl3 catalyst | Acylbenzene |
Complete oxidation | Hot, alkaline KMnO4 and then dilute acid | Benzoic acid |
Hydrogenation | Heating with hydrogen and Pt / Ni catalyst | Cyclohexane |

Unlock more, it's free!
Did this page help you?