Coloured Complexes (Cambridge (CIE) A Level Chemistry): Revision Note
Exam code: 9701
Coloured Compounds & Electron Promotion
Most transition element complexes are coloured
A transition element complex solution which is coloured, absorbs part of the electromagnetic spectrum in the visible light region
The observed colour is the complementary colour which is made up of light with frequencies that are not absorbed
For example, copper(II) ions absorb light from the red end of the spectrum
The complementary colour observed is therefore pale blue (cyan)
The visible light region of the electromagnetic spectrum

The visible light region ranges from red to violet
Electron promotion
In an isolated transition element ion (which is not bonded to any ligands), all of the 3d orbitals are degenerate
However, when ligands are attached to the central metal ion through dative covalent bonds, these orbitals are split into two sets of non-degenerate orbitals
The difference in energy between these two sets of orbitals is ΔE
When light shines on a solution containing a transition element complex, an electron will absorb this exact amount of energy (ΔE)
The amount of energy absorbed can be worked out by the equation:
ΔE = h x v
Where:
h = Planck's constant (6.626 x 10-34 m2 kg s-1)
v = frequency (Hertz, Hz or s-1)
The electron uses the energy from the light to jump into a higher, non-degenerate energy level
This is also called electron promotion
The other frequencies of light which are not absorbed combine to make the complementary colour
The diagram below shows an example of electron promotion in an octahedral complex of a nickel(II) Ni2+ ion
Electron promotion in a Ni(II) complex when light shines on the solution
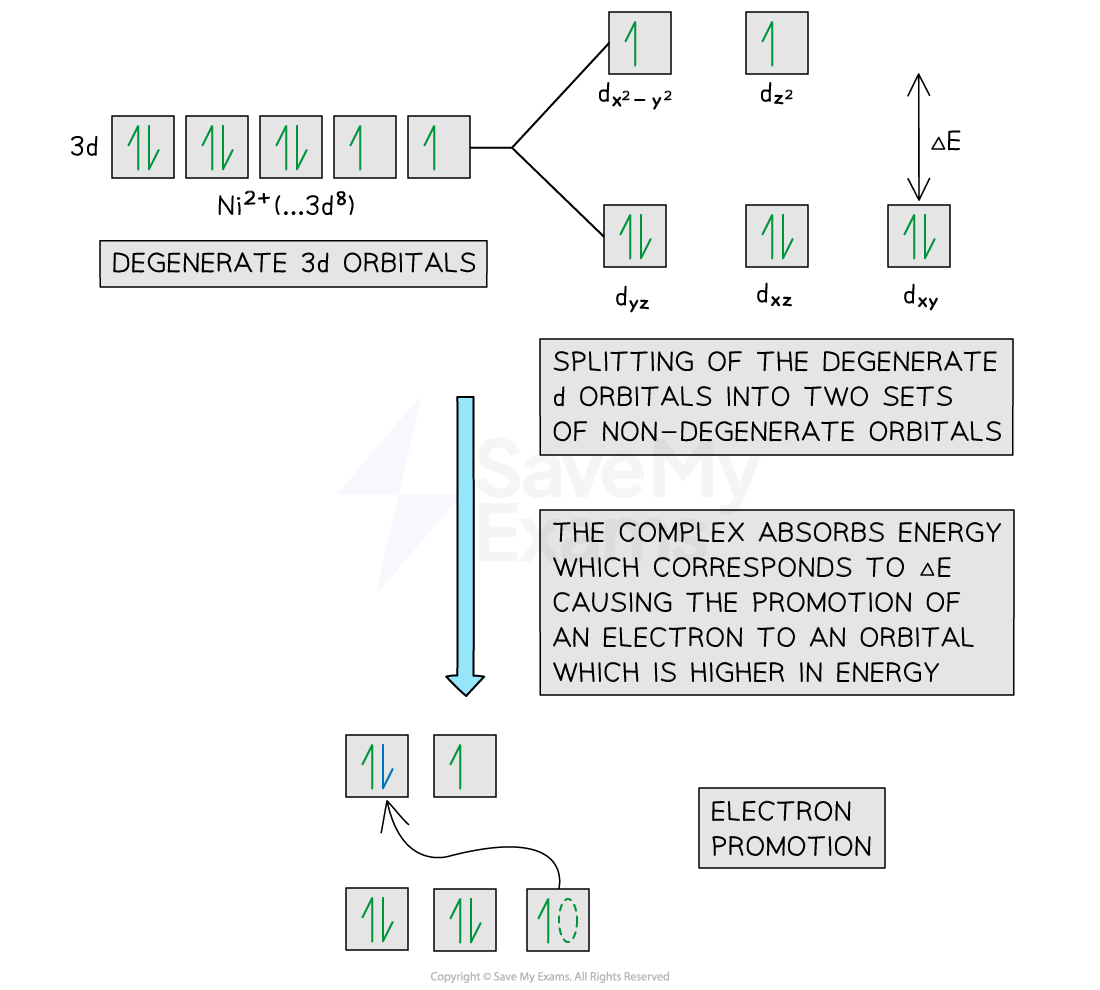
An electron gains enough energy to be promoted from a lower energy non-degenerate orbital to a higher energy non-degenerate orbital
Effects of Ligands on Complementary Colour
Transition element complexes absorb the frequency of light which corresponds to the exact energy difference (ΔE) between their non-degenerate d orbitals
The frequencies of light which are not absorbed combine to make the complementary colour of the complex
It is the complementary colour which is seen
However, the exact energy difference (ΔE) is affected by the different ligands which surround the transition element ion
Different ligands will split the d orbital by a different amount of energy
This depends on the repulsion that the d orbital experiences from these ligands
Therefore, the size of ΔE and thus the frequency of light absorbed by the electrons will be slightly different
As a result, a different colour of light is absorbed by the complex solution and a different complementary colour is observed
This means that complexes with similar transition elements ions, but different ligands, can have different colours
For example, in copper complexes:
[Cu(H2O)6]2+ complex has a light blue colour
[Cu(NH3)4 (H2O)2]2+ has a dark blue colour
Despite the copper ion having an oxidation state of +2 in both complexes
This is evidence that the ligands surrounding the complex ion affect the colour of the complex
Ligand Exchange in Copper(II) & Cobalt(II) Complexes
Different ligands may affect the complementary colour of a transition ion complex solution
This is shown by ligand exchange reactions in copper(II) and cobalt(II) complexes, as this causes a change in colour of the complexes
Copper(II) & cobalt(II) ions
The ligand exchange of [Cu(H2O)6]2+ and [Co(H2O)6]2+ by NH3 ligands causes a change in the colour of the solutions
[Cu(H2O)6]2+ is light blue in colour whereas [Cu(NH3)4(H2O)2)]2+ is deep blue in colour
[Co(H2O)6]2+ is a pink solution whereas [Co(NH3)6]2+ is a brown solution
The colour change results from the ammonia ligands, which cause the d orbitals to split by a different amount of energy (ΔE)
Therefore, the size of ΔE and the frequency of light absorbed by the electrons will be slightly different
As a result, a different colour of light is absorbed and thus a different complementary colour is observed
Ligand exchange of hexaaqua copper(II) by ammonia

Ligand exchange of the water ligands by ammonia ligands causes a change in the colour of the copper(II) complex solution from blue to dark blue
Ligand exchange of hexaaqua cobalt(II) by ammonia

Ligand exchange of the water ligands by ammonia ligand causes a change in the colour of the cobalt(II) complex solution from pink to brown
Similarly, full ligand exchange by chloride ions in copper(II) and cobalt(II) complexes results in a change in complementary colour
[Cu(H2O)4(OH)2] is a pale blue precipitate whereas [CuCl4)]2– is a yellow solution
[Co(H2O)4(OH)2] is a blue precipitate whereas [CoCl4)]2– is a blue solution
The colour change results from the chloride ligands, which cause the d orbitals to split by a different amount of energy (ΔE)
Therefore, the size of ΔE and the frequency of light absorbed by the electrons will be slightly different
As a result, a different colour of light is absorbed and thus a different complementary colour is observed
Ligand exchange of [Cu(H2O)4(OH)2] by chloride ions

Ligand exchange by chloride ligands causes a colour and state change in the colour of the copper(II) complex from a pale blue precipitate to a yellow solution
Ligand exchange of [Co(H2O)4(OH)2] by chloride ions

Ligand exchange by chloride ligands causes a colour and state change in the colour of the cobalt(II) complex from a blue precipitate to a blue solution
As before, this suggests that different ligands will split the d orbitals differently

Unlock more, it's free!
Did this page help you?