Transition Metal Complexes (Cambridge (CIE) A Level Chemistry): Revision Note
Exam code: 9701
Complex formation
What is a complex?
A complex is a molecule or ion formed by a central metal atom or ion surrounded by one or more ligands
A ligand is a species with a lone pair of electrons that can be donated to the metal ion
Ligands form dative covalent bonds with the metal by donating their lone pair of electrons
For example, [Al(H2O)6]3+ (aq):
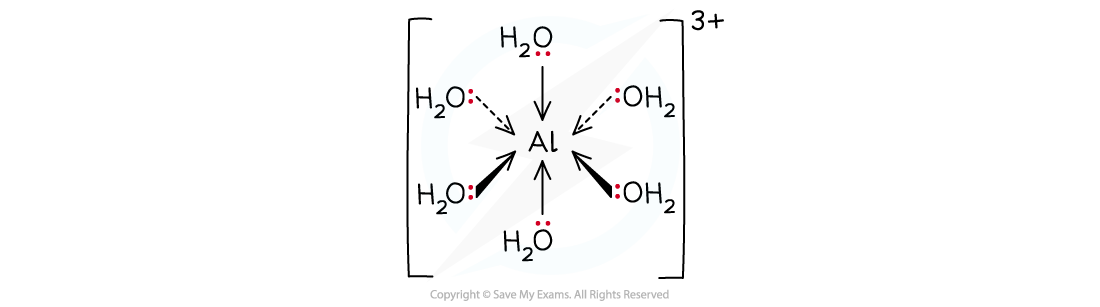
Transition metal complex formation
Transition metal ions readily form complexes with ligands
Copper(II) and cobalt(II) ions will be used as examples of the central metal ions, in the complexes with:
Water (H2O)
Ammonia (NH3)
Hydroxide (OH-)
Chloride (Cl-)
Co(II) and Cu(II) complexes with water & ammonia
Water and ammonia are neutral ligands
Water donates a lone pair from the oxygen atom
Ammonia donates a lone pair from the nitrogen atom
Water and ammonia are small ligands
Up to 6 water or ammonia ligands can fit around a central metal ion
This results in 6 dative covalent bonds
6 dative covalent bonds give:
An octahedral shape
A coordination number of 6
The coordination number of a complex is the number of dative covalent bonds formed between the central metal ion and the ligands
Cobalt(II) and copper(II) complexes with water and ammonia
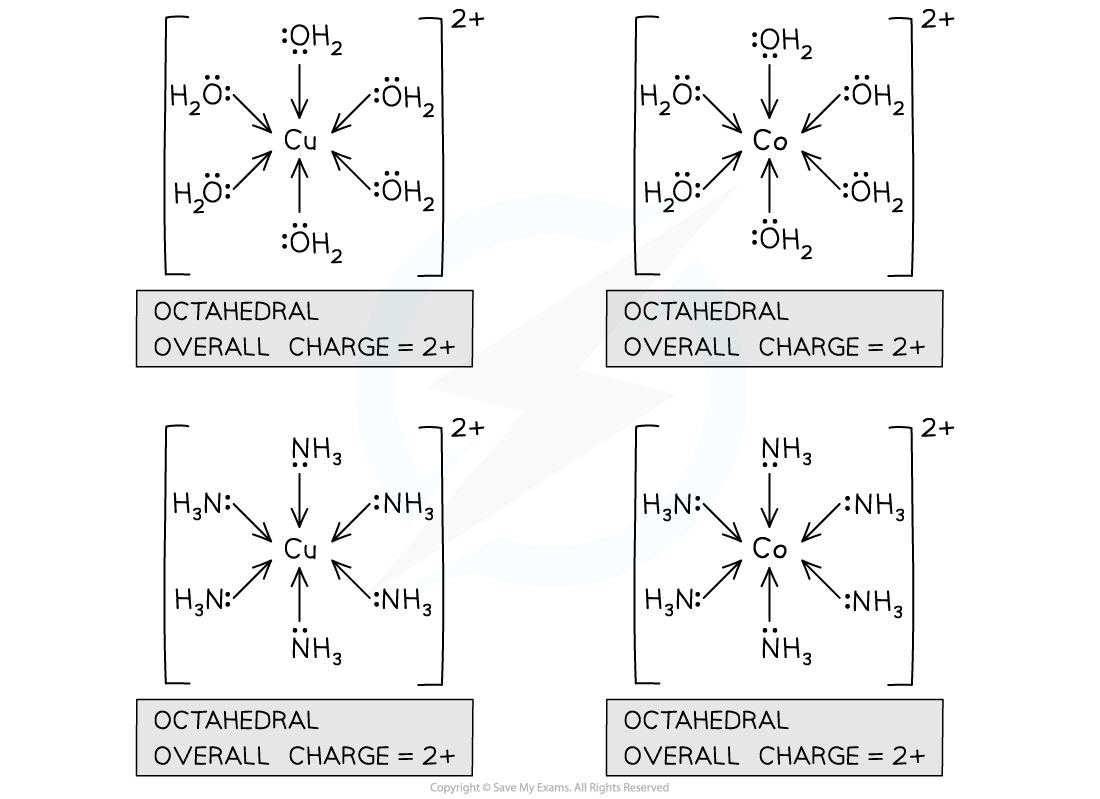
The overall charge of a complex is the sum of the charge on the central metal ion, and the charges on each of the ligands
For a cobalt(II) or copper(II) complex with 6 water or ammonia ligands:
The central metal ion has a charge of 2+
The ligands have a charge of 0
So, the overall charge of the complex is (2+) + (6 x 0) = 2+
Complexes with hydroxide & chloride ions
Hydroxide ions, OH-, and chloride ions, Cl-, are negatively charged ligands
Each donates a lone pair of electrons to form a dative covalent bond with the central metal ion
Hydroxide complexes
Hydroxide ions are small ligands
Up to 6 hydroxide ions can fit around a central metal ion
This results in:
6 dative covalent bonds
An octahedral shape
A coordination number of 6
Examiner Tips and Tricks
Although up to 6 hydroxide ions can fit around a central metal ion, in many examples only 2 hydroxide ions are present alongside 4 water ligands
Chloride complexes
Chloride ions are large ligands
Up to 4 chloride ligands can fit around a central metal ion
This results in 4 dative covalent bonds
4 dative covalent bonds give:
A tetrahedral shape
A coordination number of 4
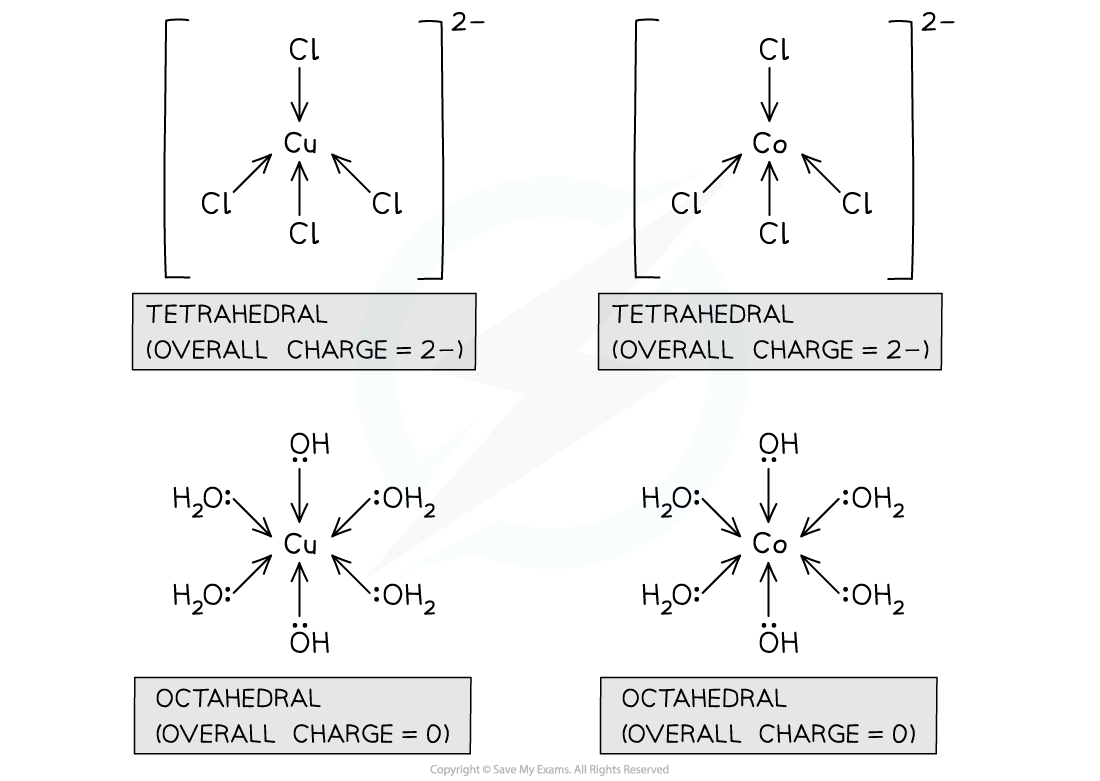
Charges of Co(II) complexes with hydroxide and chloride ligands
Co(II) ions commonly form complexes with 2 hydroxide ion ligands
The remaining ligands are water
For this cobalt(II) complex:
The central metal ion has a charge of 2+
The water ligands have a charge of 0
The 2 hydroxide ligands have a charge of 2 x (-1) = -2
So, the overall charge of the complex is (2+) + (4 x 0) + (-2) = 0
[Co(H2O)4(OH)2]
Co(II) ions commonly form complexes with 4 chloride ion ligands
For this cobalt(II) complex:
The central metal ion has a charge of 2+
The 4 chloride ligands have a charge of 4 x (-1) = -4
So, the overall charge of the complex is (2+) + (-4) = -2
[CoCl4]2-
Charges of Cu(II) complexes with hydroxide and chloride ligands
Cu(II) ions commonly form complexes with 2 hydroxide ion ligands
The remaining ligands are water
For this copper(II) complex:
The central metal ion has a charge of 2+
The water ligands have a charge of 0
The 2 hydroxide ligands have a charge of 2 x (-1) = -2
So, the overall charge of the complex is (2+) + (4 x 0) + (-2) = 0
[Cu(H2O)4(OH)2]
Cu(II) ions commonly form complexes with 4 chloride ion ligands
For this copper(II) complex:
The central metal ion has a charge of 2+
The 4 chloride ligands have a charge of 4 x (-1) = -4
So, the overall charge of the complex is (2+) + (-4) = -2
[CuCl4]2-
Comparing copper(II) and cobalt(II) complexes with chloride and water / hydroxide ions

Unlock more, it's free!
Was this revision note helpful?
