Factors affecting Rate Constant (Cambridge (CIE) A Level Chemistry) : Revision Note
Effect of Temperature on the Rate Constant
At higher temperatures, a greater proportion of molecules have energy greater than the activation energy
Since the rate constant and rate of reaction are directly proportional to the fraction of molecules with energy equal or greater than the activation energy, then at higher temperatures:
The rate constant increases
The rate of reaction increases
The relationship between the rate constant and the temperature is given by the following equation:
Where:
ln k = natural logarithm of the rate constant
A = constant related to the collision frequency and orientation of the molecules
Ea = activation energy (joules, J)
R = gas constant (8.31 J K-1 mol-1)
T = temperature (kelvin, K)
A varies only a little bit with temperature, it can be considered a constant
Ea and R are also constants
A graph of ln k against
gives a line with an equation of the form y = mx + c
Where:
y = ln k
x =
m =
(the gradient)
c = ln A (the y-intercept)
The equation shows that an increase in temperature (higher value of T) gives a greater value of ln k (and therefore a higher value of k)
Since the rate of the reaction depends on the rate constant (k) an increase in k also means an increased rate of reaction
Example graph of ln k over 1/T
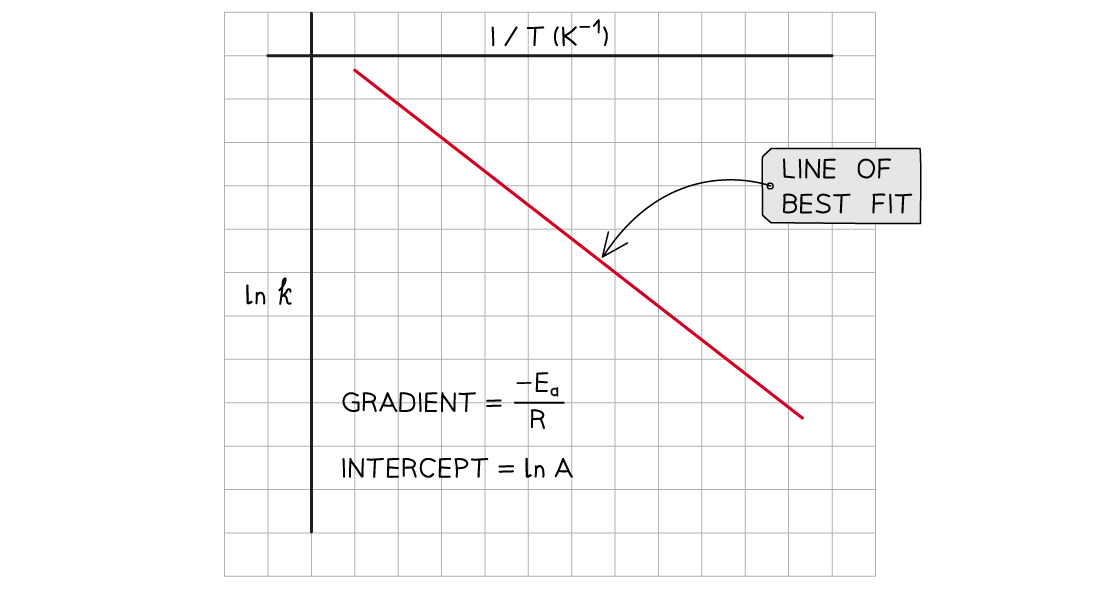
The graph of ln k over 1/T is a straight line with gradient -Ea/R
Examiner Tips and Tricks
You are not required to learn this equation however it is helpful in understanding the effects of temperature on the rate constant.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
