Constructing Born-Haber Cycles (Cambridge (CIE) A Level Chemistry)
Revision Note
Constructing Born-Haber Cycles
A Born-Haber cycle is a specific application of Hess's Law for ionic compounds and enable us to calculate lattice enthalpy which cannot be found by experiment
The basic principle of drawing the cycle is to construct a diagram in which energy increases going up the diagram
The basic principle of a Born-Haber cycle

The direction of the arrows in Born-Haber cycles indicates if a reaction is exothermic or endothermic
The cycle shows all the steps needed to turn atoms into gaseous ions and from gaseous ions into the ionic lattice
The alternative route to the ionic lattice begins from the enthalpy of formation of the elements in their standard states
Drawing the cycle for sodium chloride
A good starting point is to draw the elements with their state symbols about a third of the way up the diagram
This is shown as the left-hand side of the equation for the process indicated
The location is marked by drawing a horizontal bar or line which represents the starting energy level
Drawing a Born-Haber cycle step 1

Next, we need to create the gaseous ions
This is a two-step process of first creating the gaseous atoms and then turning them into ions
Creating gaseous atoms is a bond-breaking process, so arrows must be drawn upwards
It doesn't matter whether you start with sodium or chlorine
The enthalpy of atomisation of sodium is
Na (s) → Na (g) ΔHatθ = +108 kJ mol -1
The enthalpy of atomisation of chlorine is
½Cl2 (g) → Cl (g) ΔHatθ = +121 kJ mol -1
We can show the products of the process on the horizontal lines and the energy value against a vertical arrow connecting the energy levels
Drawing a Born-Haber cycle step 2 - creating the gaseous atoms

Now the ions are created
The sodium ion loses an electron, so this energy change is the first ionisation energy for sodium
Na (g) → Na+ (g) + e– ΔHieθ = +500 kJ mol-1
The change is endothermic so the direction continues upwards
The chlorine atom gains an electron, so this is electron affinity
Cl (g) + e– → Cl- (g) ΔHeaθ = -364 kJ mol-1
The exothermic change means this is downwards
The change is displaced to the right to make the diagram easier to read
Drawing a Born-Haber cycle step 3 - creating the gaseous ions

The two remaining parts of the cycle can now be completed
The enthalpy of formation of sodium chloride is added at the bottom of the diagram
Na(s) + ½Cl2 (g) → NaCl (s) ΔHfθ = -411 kJ mol -1
This is an exothermic change for sodium chloride so the arrow points downwards
Enthalpy of formation can be exothermic or endothermic, so you may need to show it above the elements ( and displaced to the right) for a endothermic change
The final change is lattice enthalpy, which is usually shown a formation. For sodium chloride the equation is
Na+(g) + Cl-(g) → NaCl (s) ΔHlattθ
Drawing a Born-Haber cycle step 4 - completing the cycle

The cycle is now complete
The cycle is usually used to calculate the lattice enthalpy of an ionic solid, but can be used to find other enthalpy changes if you are given the lattice enthalpy
Worked Example
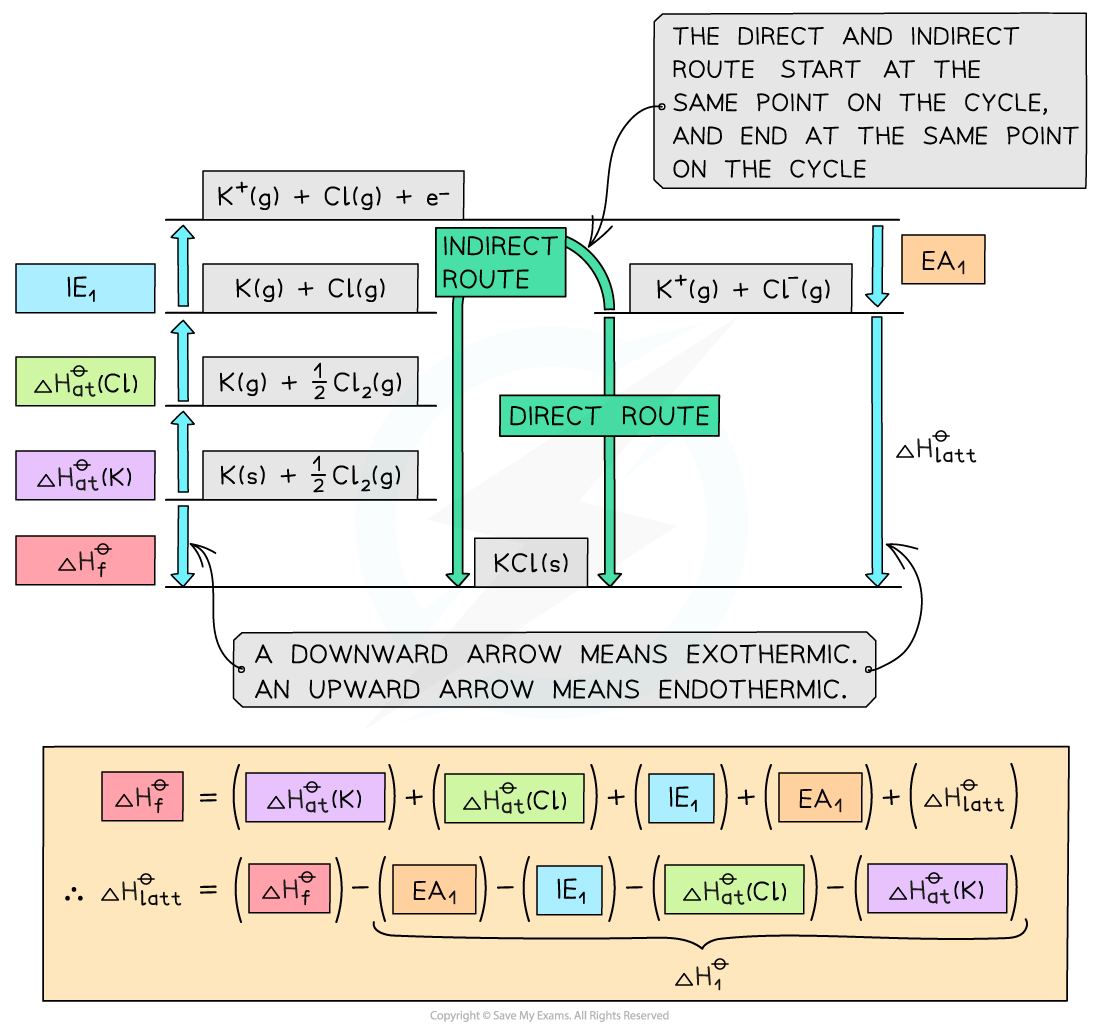
Construct a Born-Haber Cycle which can be used to calculate the lattice energy of potassium chloride.
Step | Equation | Enthalpy Change |
|---|---|---|
Convert K (s) atoms into K (g) atoms | K (s) → K (g) | ΔHatθ |
Convert K (g) atoms into K+ (g) ions | K (g) → K+ (g) | IE1 |
Convert Cl2 (g) molecules into Cl (g) atoms | ½Cl2 (g) → Cl (g) | ΔHatθ |
Convert Cl (g) atoms into Cl– (g) ions | Cl (g) + e– → Cl– (g) | EA1 |
Add up all values to get ΔH1θ |
| ΔH1θ |
Apply Hess's Law to find ΔHlattθ |
| ΔHlattθ |
Answer:
Worked Example
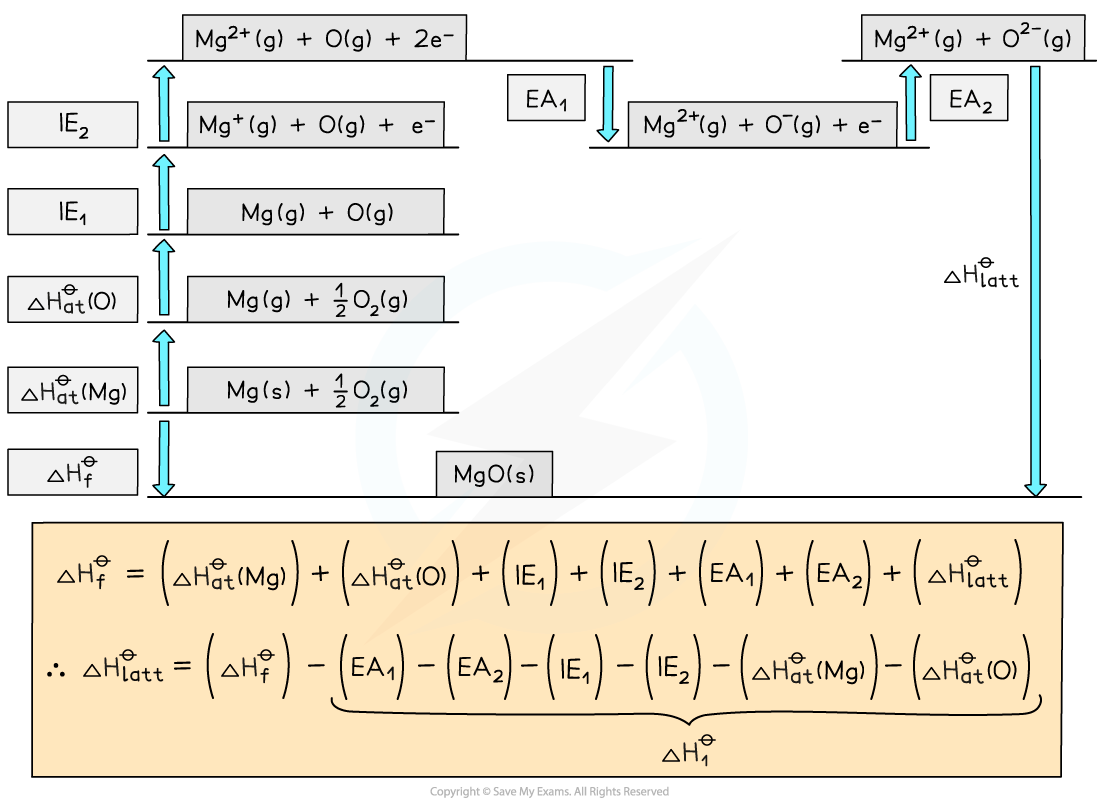
Construct a Born-Haber Cycle which can be used to calculate the lattice energy of magnesium oxide.
Step | Equation | Enthalpy Change |
|---|---|---|
Convert Mg (s) atoms into Mg (g) atoms | Mg (s) → Mg (g) | ΔHatθ |
Convert Mg (g) atoms into Mg+ (g) ions | Mg (g) → Mg+ + e– (g) | IE1 |
Convert Mg+ (g) atoms into Mg2+ (g) ions | Mg+ (g) → Mg2+ + e– (g) | IE2 |
Convert O2 (g) molecules into O (g) atoms | ½O (g) → O (g) | ΔHatθ |
Convert O (g) atoms into O– (g) ions | O (g) + e– → O– (g) | EA1 |
Convert O– (g) atoms into O2– (g) ions | O– (g) + e– → O2– (g) | EA2 |
Add up all values to get ΔH1θ |
| ΔH1θ |
Apply Hess's Law to find ΔHlattθ |
| ΔHlattθ |
Answer:

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
