Combustion of Alkanes (Cambridge (CIE) A Level Chemistry) : Revision Note
Combustion of Alkanes & the Environment
Cars’ exhaust fumes include toxic gases such as carbon monoxide (CO), oxides of nitrogen (NO/NO2) and volatile organic compounds (VOCs)
When released into the atmosphere, these pollutants have drastic environmental consequences damaging nature and health
Carbon monoxide
Carbon monoxide is formed in the incomplete combustion of alkanes inside a car engine
Due to a lack of enough oxygen in the engine, some of the carbon is only partially oxidised to CO instead of carbon dioxide (CO2)
Word equation for incomplete combustion

Incomplete combustion of alkanes is caused by a limited supply of oxygen
CO is a toxic and odourless gas which can cause dizziness, loss of consciousness and eventually death
The CO binds to haemoglobin which therefore cannot bind oxygen and carbon dioxide
Oxygen is transported to organs
Carbon dioxide is removed as waste material from organs
The effect of carbon monoxide on haemoglobin
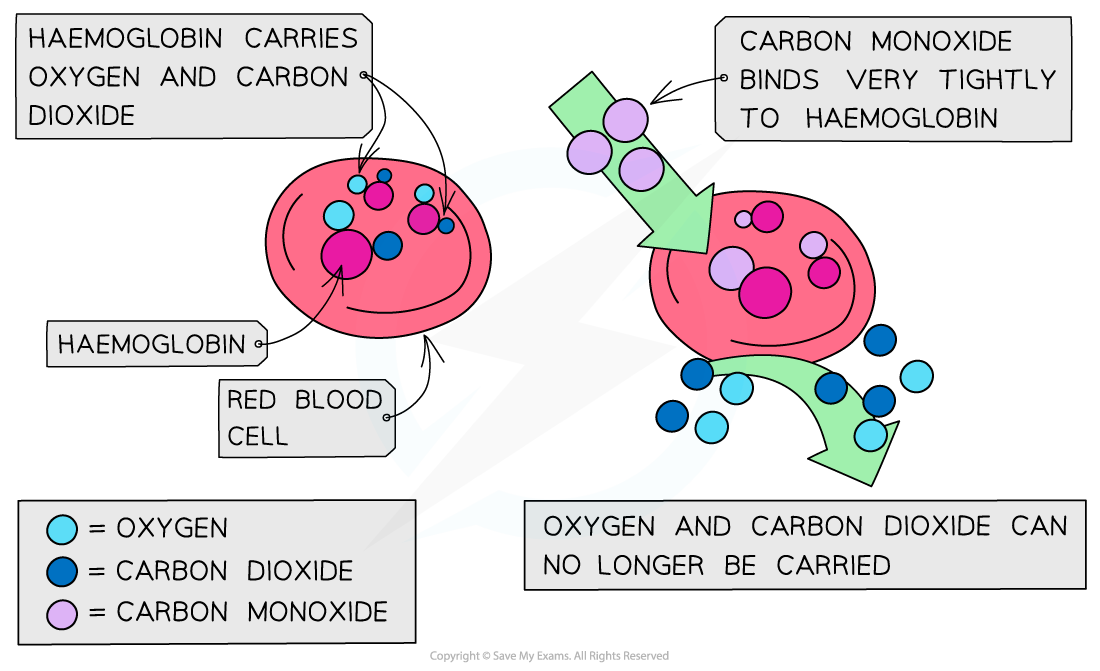
The high affinity of CO to haemoglobin prevents it from binding to O2 and CO2
Oxides of nitrogen
Normally, nitrogen is too unreactive to react with oxygen in air
However, in a car’s engine, high temperatures and pressures are reached causing the oxidation of nitrogen to take place:
N2 (g) + O2 (g) → 2NO (g)
N2 (g) + 2O2 (g) → 2NO2 (g)
The oxides of nitrogen are then released in the car’s exhaust fumes into the atmosphere
Car exhaust fumes also contain unburnt hydrocarbons from fuels and their oxides (VOCs)
In the air, the nitrogen oxides can react with these VOCs to form peroxyacetyl nitrate (PAN) which is the main pollutant found in photochemical smog
PAN is also harmful to the lungs, eyes and plant life
Nitrogen oxides can also dissolve and react in water with oxygen to form nitric acid which is a cause of acid rain
Acid rain can cause corrosion of buildings, endanger plant and aquatic life (as lakes and rivers become too acidic) and directly damage human health
Catalytic removal
To reduce the amount of pollutants released in cars’ exhaust fumes, many cars are now fitted with catalytic converters
Precious metals (such as platinum) are coated on a honeycomb to provide a large surface area
The reactions that take place in the catalytic converter include:
Oxidation of CO to CO2:
2CO + O2 → 2CO2
or
2CO + 2NO → 2CO2 + N2
Reduction of NO/NO2 to N2:
2CO + 2NO → 2CO2 + N2
Oxidation of unburnt hydrocarbons:
CnH2n+2 + (3n+1)[O] → nCO2 + (n+1)H2O
Pollutants, their effect & removal table
| Formation | Environmental Consequence | Catalytic Removal |
|---|---|---|---|
Carbon Monoxide | Incomplete combustion of alkanes in car engines | Toxic gas | Oxidation to CO2 2CO + O2 → 2CO2 OR 2CO + 2NO → 2CO2 + N2 |
Oxides of Nitrogen | Oxidation of nitrogen in car engines | Dissolve in and react in water with oxygen to form acid rain | Reduction to nitrogen gas: 2CO + 2NO → 2CO2 + N2 |
VOCs | Unburnt hydrocarbons from fuels and their oxides formed in car engines | React with oxides of nitrogen in the atmosphere to produce PAN | Oxidise unburnt hydrocarbons to carbon dioxide and water CnH2n+2 + (3n + 1)[O] → nCO2 + (n + 1)H2O |
PAN | From the photochemical reaction of VOCs and nitrogen oxides in the atmosphere | Photochemical smog | Oxidise unburnt hydrocarbons and reduce nitrogen oxides to prevent the formation of PAN in the atmosphere |
Examiner Tips and Tricks
Although CO2 is not a toxic gas, it is still a pollutant causing global warming and climate change

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
