This question is about the formation of calcium oxide.
Explain the meaning of the term lattice energy.
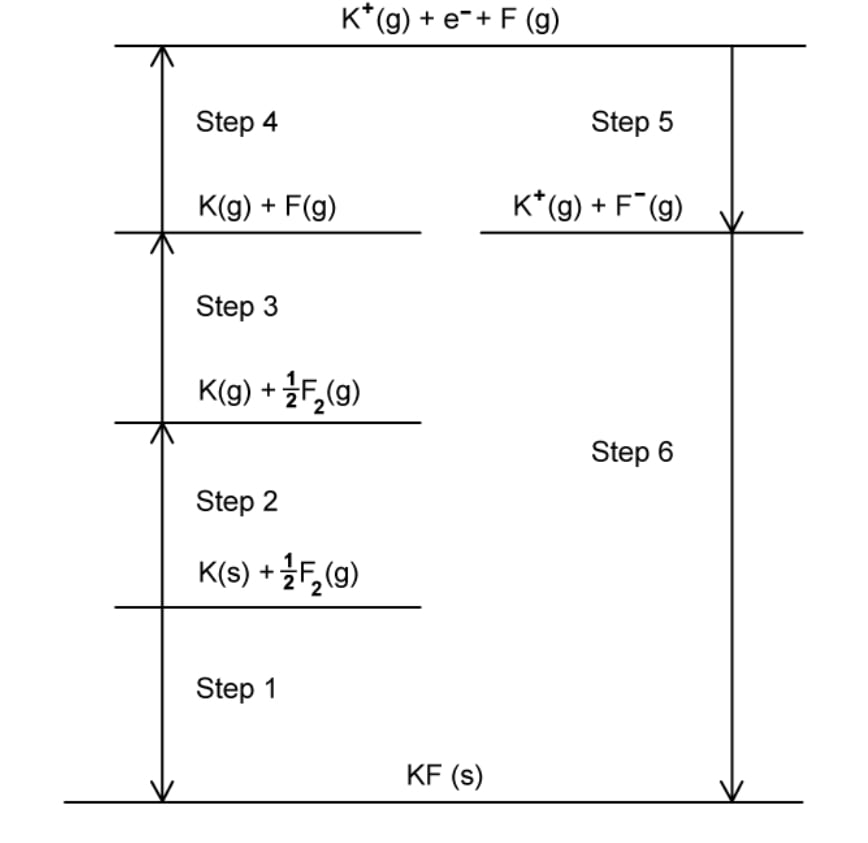
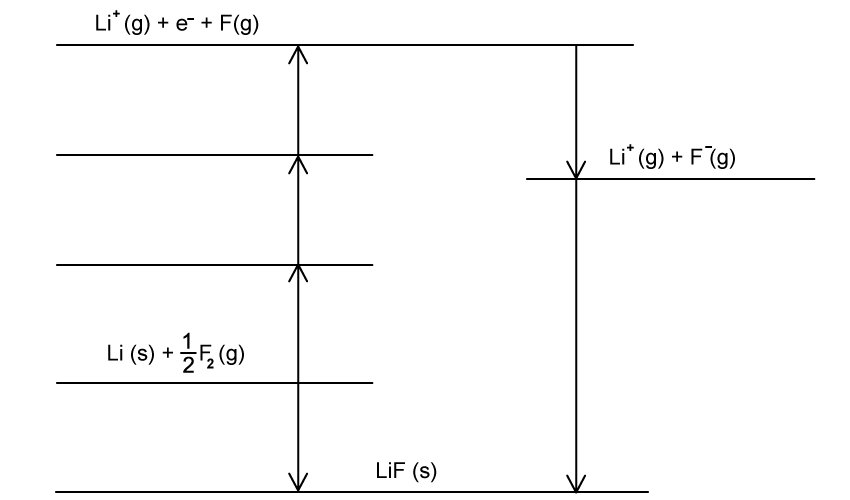
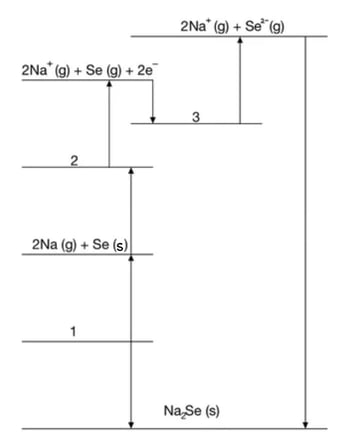
The Born- Haber cycle In Fig. 1.1 can be used to determine the lattice enthalpy of calcium oxide.
Steps A-G includes the values for enthalpy changes.
Complete the Born Haber cycle by adding the species present on the two dotted lines.
Include state symbols.
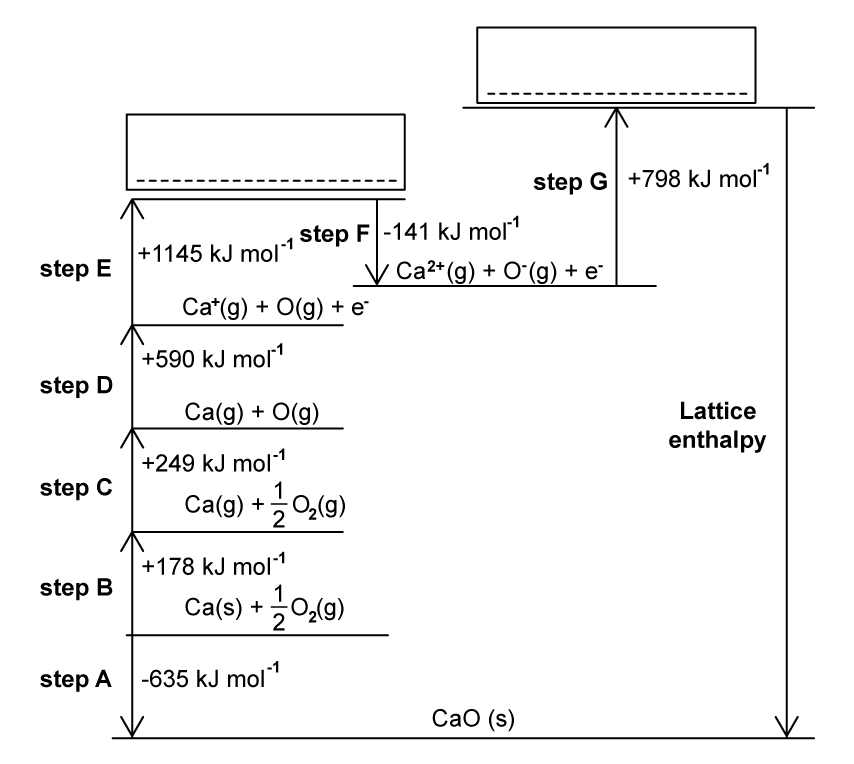
Name the enthalpy changes for the following steps in the Born-Haber cycle.
- Step B .....................................................................
- Step D ...................................................................
- Step F ...................................................................
Step C represents the enthalpy change of atomisation of oxygen.
Why is the enthalpy change of atomisation always a positive value?
Did this page help you?