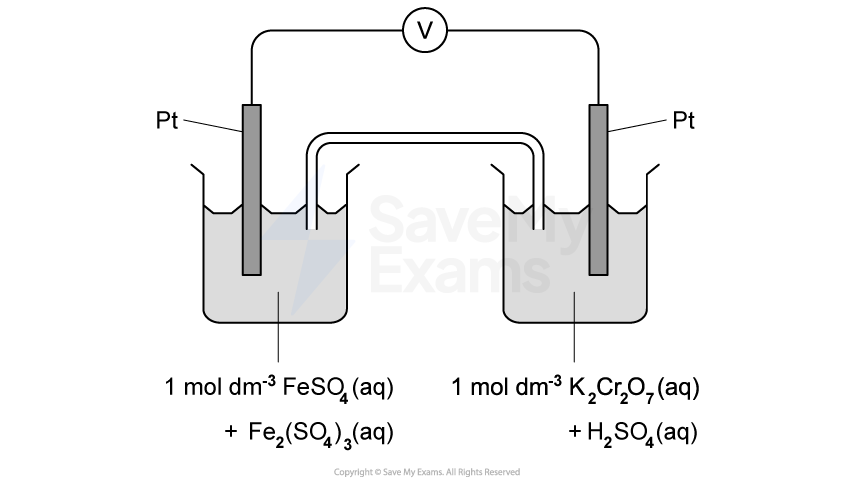
State the standard conditions for measurements in electrochemical cells.
Figure 1 shows a half cell that can be used to calculate the standard electrode potential of the Fe2+ / Fe reaction.
Figure 1

Write the half equation, including state symbols, that represents this half cell.
A standard hydrogen electrode can be connected to the Fe2+ / Fe electrode to measure the standard electrode potential, EӨ.
Using the information in part (b), draw the labelled, electrochemical cell that is used to measure the standard electrode potential of the Fe2+ / Fe electrode.
Write the cell representation for the electrochemical cell set up in parts (b) and (c), using the standard hydrogen electrode and the Fe2+ / Fe electrode.
Was this exam question helpful?