Lattice enthalpy is a measure of the strength of the forces between the ions in an ionic solid.
i) Give the definition of the term enthalpy of lattice formation.
ii) Write one equation to represent each the following changes:
Atomisation of sodium
Second ionisation energy of magnesium
First electron affinity of chlorine
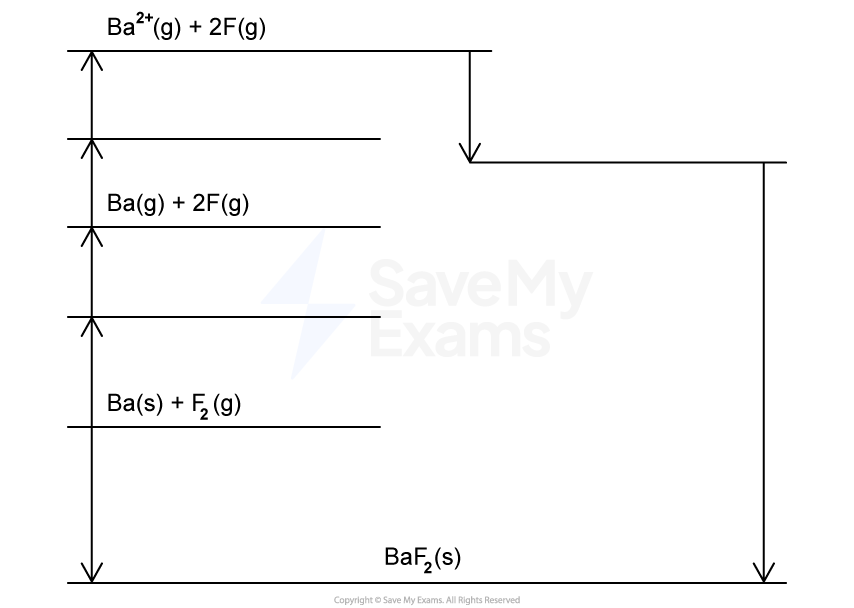
Complete Table 1 for the following Born-Harber cycle for formation of potassium fluoride shown in Figure 1.
Figure 1

Table 1
Step | Name of the Enthalpy Change |
1 |
|
2 | Atomisation of potassium |
3 |
|
4 | First ionisation energy of potassium |
5 |
|
6 | Lattice enthalpy of formation |
The enthalpy of lattice formation of potassium fluoride and caesium fluoride is -830 kJ mol-1 and -730 kJ mol-1 respectively.
Explain why the enthalpy of lattice formation is more exothermic for potassium fluoride.
Use the data in Table 2 to calculate the enthalpy of solution of potassium fluoride.
Table 2
ΔHӨlattKF (kJ mol-1) | +830 |
ΔHӨhydK+ (kJ mol-1) | -351 |
ΔHӨhydF- (kJ mol-1) | -504 |
Did this page help you?