State the definition of dynamic equilibrium.
State the meaning of a closed system.
State Le Chatelier's principle.
Write the expression for Kc for the following reaction:
2A + B → 3C + D
Did this page help you?
Exam code: 7405
Select a download format for Chemical Equilibria, Le Chatelier’s Principle & Kc
Select an answer set to view for
Chemical Equilibria, Le Chatelier’s Principle & Kc
State the definition of dynamic equilibrium.
How did you do?
State the meaning of a closed system.
How did you do?
State Le Chatelier's principle.
How did you do?
Write the expression for Kc for the following reaction:
2A + B → 3C + D
How did you do?
Did this page help you?
Sulfur trioxide, SO3, decomposes to establish an equilibrium producing sulfur dioxide, SO2, and oxygen as shown in the reaction.
2SO3 (g) 2SO2 (g) + O2 (g) ΔH = + 196 kJ mol-1
i) State whether the forward reaction is endothermic or exothermic.
ii) State the effect on the yield of sulfur dioxide if the temperature of the reaction is increased.
How did you do?
The pressure of the dynamic equilibrium outlined in part (a) is increased.
i) State the effect of increasing the pressure on the yield of sulfur dioxide, SO2.
ii) Explain your answer to part (i).
How did you do?
Give the expression for Kc for the reaction outlined in part (a).
How did you do?
For the reaction outline in part (a), at dynamic equilibrium, the concentrations of each compound are given in Table 1 when the temperature is 600 C.
Table 1
| SO3 | SO2 | O2 |
Concentration at equilibrium (mol dm-3) | 0.093 | 0.100 | 0.200 |
Calculate the value of Kc to 3 significant figures.
How did you do?
Did this page help you?
Hydrogen gas, H2, is produced by passing methane, CH4 and steam, H2O over a heated catalyst. This is known as steam-methane reforming (SMR). The reaction for the process is shown below:
CH4 (g) + H2O (g) CO (g) + 3H2 (g) ΔH = + 206 kJ mol-1
A chemist carries out the reaction at 800 C and at 30 atm.
Use Le Chatelier's principle to explain why a high temperature is favourable for this process.
How did you do?
Use le Chatelier's principle to explain why a low pressure is favourable for this process.
How did you do?
Give the expression for Kc for the reaction outlined in part (a).
How did you do?
State the units for Kc for the reaction outlined in part (a).
How did you do?
Did this page help you?
The reaction below shows the decomposition of dinitrogen tetroxide, N2O4, into two molecules of nitrogen dioxide, NO2.
N2O4 (g) → 2NO2 (g) ΔH = + 58 kJ mol-1
A dynamic equilibrium is reached at a temperature of 298K. The concentrations of each of the compounds at equilibrium are shown in Table 1.
Table 1
| N2O4 | NO2 |
Concentration at equilibrium (mol dm-3) | 0.0647 | 0.0206 |
Give the expression for Kc for this reaction.
How did you do?
Calculate a value for Kc to three significant figures.
How did you do?
State the units for Kc for the reaction outlined in part (a).
How did you do?
At the start of the reaction outlined in part (a) dinitrogen tetroxide, N2O4, is the only compound present.
Sketch two lines on the graph shown in Figure 1 to show the change in concentration for both dinitrogen tetroxide, N2O4, and nitrogen dioxide, NO2 as the reaction reaches dynamic equilibrium.
You should make reference to the information given in Table 1.
Figure 1

How did you do?
Did this page help you?
Phosphorus trichloride, PCl3, and oxygen, O2, are reacted to produce phosphorus oxychloride, POCl3.
2PCl3 (g) + O2 (g) 2POCl3 (g) ΔH = -153.6 kJ mol-1
All the compounds in the equilibrium are in the gaseous state.
State the name of the type of equilibrium in which all reactants and products in the mixture are in the same state.
How did you do?
Using Le Chatelier's principle, state and explain the effect that increasing the temperature will have on the yield of phosphorus oxychloride, POCl3.
How did you do?
Using Le Chatelier's principle, state and explain the effect that increasing the pressure will have on the yield of phosphorus oxychloride, POCl3.
How did you do?
When hydrogen and bromine gas are reacted together at 573 C the value of the equilibrium constant, Kc, is 4.1 x 1018. A dynamic equilibrium is established. This reaction is shown below:
H2 (g) + Br2 (g) 2HBr (g)
State and explain the effect, if any, of decreasing the pressure on the value of the equilibrium constant, Kc.
How did you do?
Did this page help you?
Ethanol has a great number of uses. For industrial purposes, it can be manufactured via the following reversible reaction.
C2H4 (g) + H2O (g) C2H5OH (g) ?H = -46 kJ mol-1
The optimum pressure for this reaction is between 60 and 70 atm.
Using Le Chatelier’s principle, state and explain the effect, if any, that increasing the overall pressure would have on the equilibrium yield of ethanol.
How did you do?
Although Le Chatelier’s principle is used to suggest the best conditions for a reaction, often a compromise has to be made.
i) Use Le Chatelier’s principle to suggest whether a high or low temperature should be used to produce the maximum yield of ethanol in the reaction from part (a).
ii) State a problem which may occur from using this temperature.
How did you do?
Ethanol can be used as a reactant in another equilibrium reaction; the manufacture of ethyl ethanoate.
CH3CH2OH (l) + CH3COOH (l) CH3COOCH2CH3 (l) + H2O (l)
Give the expression for the equilibrium constant, Kc, for this equilibrium.
How did you do?
A student set up the esterification reaction seen in part (c), adding ethanol and ethanoic acid to a reaction vessel. They set the reaction up in a closed system, at a constant temperature and allowed equilibrium to be reached.
The reaction was done in a container with a volume of 250 cm3.
Table 1 below shows the amount of each substance present in the equilibrium mixture.
Table 1
Substance | Amount (mol) |
CH3CH2OH | 0.0375 |
CH3COOH | 0.0615 |
CH3COOCH2CH3 | 0.0776 |
H2O | 0.0834 |
i) Calculate Kc for this reaction to 2 decimal places.
ii) Deduce the units for Kc.
How did you do?
Did this page help you?
Methanol is the first member of the alcohol homologous series. It is an essential industrial chemical, and can be manufactured according to the following reaction:
CO2 (g) + 3H2 (g) CH3OH (g) + H2O (g) ?H = -91 kJ mol-1
The conditions used are called compromise conditions, used to find a balance between producing a high yield of product and having manageable and safe conditions.
Give the Kc expression for this equilibrium reaction and deduce the units.
How did you do?
The reaction in part (a) often uses a copper-based catalyst, meaning that the usual temperature and pressure of the reaction can be reduced.
State and explain the effect, if any, that using a catalyst for this reaction will have on the yield of methanol which is produced.
How did you do?
State and explain why lowering the temperature of the reaction will contribute to a higher yield of methanol being produced, but why lowering the pressure would contribute to a lower yield of product.
How did you do?
Excluding any reasons regarding the yield of the product, give a reason why scientists in industry much prefer to use a lower pressure and temperature where possible.
How did you do?
Did this page help you?
Some industrial processes involve reversible reactions. If done under the correct conditions, then these reactions will reach a dynamic equilibrium, producing equilibrium mixtures of reactants and products. The conditions of the reaction can be altered and controlled to maximise the product yield.
Le Chatelier’s principle is a rule which is used to determine how to move the equilibrium position to the left or right, by altering specific conditions of the reaction.
State Le Chatelier’s principle.
How did you do?
An example of an equilibrium reaction can be seen between oxygen and nitrogen.
When heated to a high enough temperature, nitrogen will react with oxygen to form nitrogen monoxide, as shown below:
N2 (g) + O2 (g) 2NO (g)
When the temperature of the system is increased, the yield of NO increases.
State whether the forward reaction in this system is exothermic or endothermic.
Explain your answer.
How did you do?
State and explain the effect, if any, on the yield of NO produced if the pressure was increased, but the temperature was kept the same.
How did you do?
A chemist set up a different reaction, shown below:
W (aq) + 2X (aq) 3Y (aq) + Z (aq)
They started with a flask containing 1.75 x 10-2 mol of an aqueous solution W. They then added 0.050 mol of solution X to the flask, ensured a closed system, and allowed the reaction to reach equilibrium.
Once equilibrium was reached, the reaction mixture contained 0.012 mol of Z.
The overall volume of the reaction mixture was 105 cm3.
i) Give the Kc expression for this reaction.
ii) Calculate a value for Kc.
How did you do?
Did this page help you?
A reaction mixture was set up in a syringe between dinitrogen tetraoxide gas and nitrogen dioxide gas as shown in the equation below:
N2O4 (g) 2NO2 (g) ?H = +58 kJ mol-1
The appearance of the gases is very different; dinitrogen tetraoxide is a colourless gas, whereas nitrogen dioxide is dark brown in colour.
Give the Kc expression for this reaction and deduce the units.
How did you do?
Explain why the reaction mixture turns darker in colour when it is heated.
How did you do?
The reaction which takes place in part (a) has a Kc value of 3.21. A student claims that increasing the temperature of this reaction will increase the value of Kc.
Is the student correct? Justify your answer.
How did you do?
Using Le Chatelier’s principle, explain what would be seen initially if the plunger of the syringe was pressed and the gases within the syringe were compressed.
How did you do?
Did this page help you?
During an esterification reaction, methanol and ethanoic acid react together to form the ester, methyl ethanoate, and water as shown below:
CH3OH (l) + CH3COOH (l) CH3COOCH3 (l) + H2O (l)
A chemist sets up the reaction and allows it to reach dynamic equilibrium at a constant temperature.
i) State the meaning of the term dynamic equilibrium.
ii) Give one key condition which must be satisfied for a reversible reaction to reach dynamic equilibrium.
How did you do?
Once the reaction in part (a) is set up, the students leave it for 24 hours to make sure that it has reached equilibrium.
State how the students could check to make sure that the reaction mixture had reached equilibrium.
How did you do?
When the equilibrium was reached, the equilibrium moles of each component in the mixture were as follows:
- Ethanoic acid = 0.378
- Methanol = must be calculated
- Methyl ethanoate = 0.716
- Water = 1.08
Kc for the reaction was 7.21.
i) Calculate the number of moles of methanol at equilibrium.
ii) State why there are no units for Kc in this reaction.
How did you do?
Adding more ethanoic acid to the reaction mixture will increase the yield of the ester produced.
Use Le Chatelier’s principle to explain the above statement.
How did you do?
Did this page help you?
The Haber process allows ammonia to be manufactured on a large scale for use in fertilisers.
The balanced equation for the Haber process is shown below.
N2 (g) + 3H2 (g) 2NH3 (g)
The equilibrium percentage of ammonia depends on the temperature as shown in Figure 1.
Figure 1
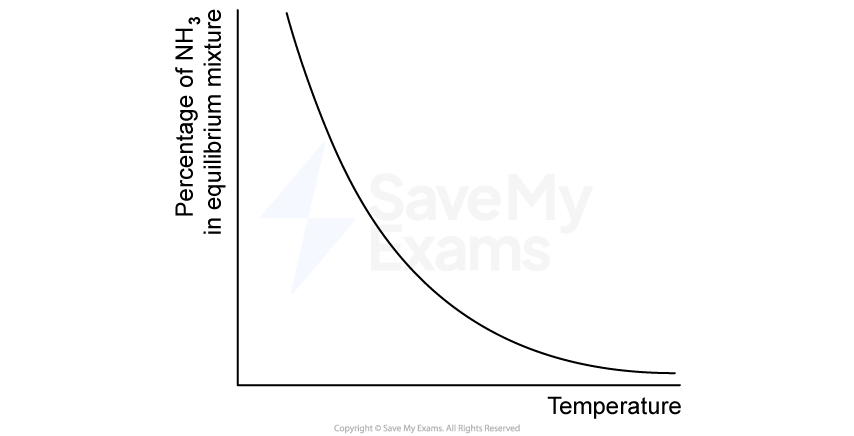
Use Figure 1 to determine whether the forward reaction is exothermic or endothermic, and justify your answer.
Describe and explain how raising the pressure affects the yield of ammonia.
Write the expression for the equilibrium constant, Kc, for this reaction.
How did you do?
A 1.0 dm3 flask at 400 oC was filled with 1.00 mol of N2 and 3.00 mol of H2.
At equilibrium, the concentration of NH3 was measured as 0.062 mol dm-3.
Calculate the value of the equilibrium constant, Kc, for the reaction under these conditions.
How did you do?
In the Haber process, iron acts as a catalyst.
State how the presence of a catalyst affects the value of Kc.
How did you do?
The effect of a catalyst on activation energy can be represented in a Maxwell-Boltzmann distribution curve, shown in Figure 2.
Figure 2.
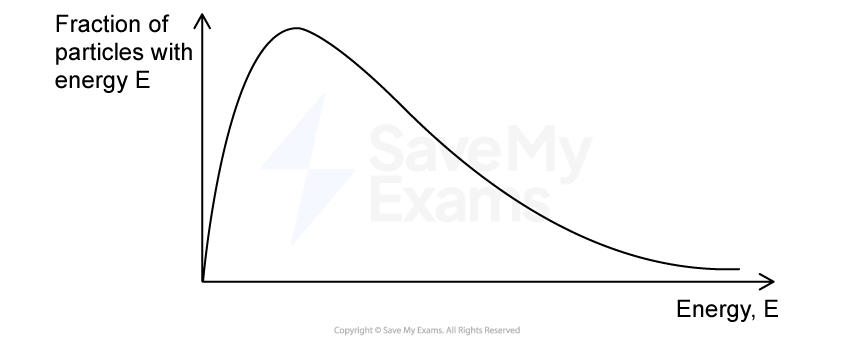
Complete the diagram by showing the location of the activation energy (Ea) with and without a catalyst.
How did you do?
Did this page help you?
The following dynamic equilibrium was reached at temperature, T, in a closed container.
2 X (g) + Y (g) 2 Z (g) ΔH = - 65 kJ mol-1
The value of Kc for the reaction was 75.0 mol-1 dm3 when the equilibrium mixture contained 2.97 mol of Y and 5.38 mol of Z.
i) Give the definition of dynamic equilibrium.
ii) Write an expression for Kc for the reaction.
iii) Show how the units for Kc are mol-1 dm3.
How did you do?
Calculate the concentration of X in the equilibrium mixture if the volume of the container is 12.00 dm3. Give your answer to 3 significant figures.
How did you do?
If the conditions for a closed container are changed, it can have an effect on the concentrations of the reactants, products and Kc.
State the effect, if any, on the concentration of Y at equilibrium if temperature, T, is decreased and give a reason for your answer.
How did you do?
Calculate the equilibrium constant for the following reaction at temperature, T.
2 Z (g) 2 X (g) + Y (g)
How did you do?
Did this page help you?
A 0.680 mol sample of SO3 is introduced into a 3.04 dm3 reaction container and allowed to reach equilibrium at temperature T. 32% of the SO3 had decomposed.
Calculate the value for Kc in this reaction, giving your answer to 2 significant figures.
2 SO3 (g) 2 SO2 (g) + O2 (g) ΔH = + 196 kJ mol-1
How did you do?
The size of the container for the reaction in part (a) is increased. State the effect if any on the equilibrium constant, Kc, and the position of equilibrium. Justify your answer.
How did you do?
The temperature of the reaction in part (a) is increased. State the effect, if any, on the equilibrium constant, Kc, and the position of equilibrium. Justify your answer.
How did you do?
If the value of the equilibrium constant, Kc, is 2.7 x 10-2 at temperature T1 for the reaction:
2 SO3 (g) 2 SO2 (g) + O2 (g)
i) Calculate the equilibrium constant, Kc, for the reaction:
4 SO2 (g) + 2 O2 (g) 4 SO3 (g)
Give your answer to 2 decimal places.
ii) State the units for Kc for the reaction in part (i).
How did you do?
Did this page help you?
A mixture of 1.32 moles of E, 1.49 moles of F and 0.752 moles of G were placed into a 5.0 dm3 container at temperature, T, and allowed to reach equilibrium. At equilibrium, the number of moles of E was 1.86.
Calculate the value of the equilibrium constant, Kc, to 3 significant figures.
2 E (g) 2 F (g) + G (g) ΔH = -143 kJ mol-1
How did you do?
The value of Kc for the reaction in part (a) at a different temperature, T1, is 2.98 mol dm-3. Comment on the relationship between the concentration of the reactant E and products F and G with regards to Kc.
How did you do?
Reactants G and H react together to form products J and K according to the equation
3G + H 4J + K
A beaker contained 35 cm3 of 0.18 mol dm-3 of an aqueous solution of G.
8.41 x 10-3 moles of H and 3.1 x 10-3 moles of J were also added to the beaker. The equilibrium mixture contained 4.1 x 10-3 moles of G.
Calculate the number of moles of H, J and K at equilibrium.
How did you do?
For the reaction in part (c) write the expression for the equilibrium constant, Kc, and state the units.
How did you do?
Did this page help you?
Diesters are compounds often used as synthetic lubricants for machinery such as compressors. The reaction below shows the formation of a diester from propanoic acid and propane-1,3-diol.
2 CH3CH2COOH + HOCH2CH2CH2OH C9H16O4 + 2 H2O
At equilibrium the reaction mixture contained 3.25 moles of CH3CH2COOH, 1.15 moles of HOCH2CH2CH2OH, 1.18 moles of C9H16O4.
The value for Kc at temperature, T, is 1.29.
Give the units for Kc.
How did you do?
Calculate the concentration of water in the reaction mixture in part (a) at equilibrium. Give your answer to 3 significant figures.
How did you do?
A student deduced that in order to calculate the value of Kc for the reaction in part (a) you must work out the concentrations using the overall volume.
Justify whether the student is correct.
How did you do?
The forward reaction in part (a) is slightly exothermic. At a different temperature, T1, the value for Kc increases to 22.78.
State whether the new temperature, T1, is higher or lower than the original temperature. Justify your answer.
How did you do?
Did this page help you?
The graph in Figure 1 shows the effect on pressure and temperature on the equilibrium yield of gaseous molecules.
Figure 1

Use Figure 1 to fully explain whether the forward reaction is exothermic or endothermic
How did you do?
Use Figure 1 to fully explain whether the forward reaction will involve either an increase or decrease in the number of moles of a gas.
How did you do?
The graph to show the relationship between temperature and Kc for a different dynamic equilibrium to produce a gaseous product is shown in Figure 2.
Figure 2

Use the information in Figure 2 to establish whether the forward reaction is exothermic or endothermic. Justify your answer.
How did you do?
Gaseous products can be manufactured by the direct combination of reactants.
Explain why it is important for a chemist to know the value of Kc, at a given temperature, for such a reaction.
How did you do?
Did this page help you?