Colorimetry & Complex Ions (AQA A Level Chemistry) : Revision Note
Colorimetry & Complex Ions
Colorimetry & Complex Ions
Colorimetry can be used to measure the absorption of radiation using a colorimeter
This uses a lamp as a source of white light, which passes through a filter to produce light of one colour
This colour will be the one that the sample will absorb the most and is called its complementary colour
For example hexaaquacopper(II) solution is a pale blue colour which absorbs red light
It's complementary colour is blue which is what is transmitted

A simple diagram representing the component parts involved in colorimetry
Worked Example
Outline a practical method for the preparation of an unknown concentration of metal aqua ion solution by colorimetry.
Answer:
Make up metal aqua ion solutions of known concentrations
Measure absorption or transmission
Plot a calibration curve (absorbance vs concentration)
Measure absorption of unknown
Determine concentration of unknown
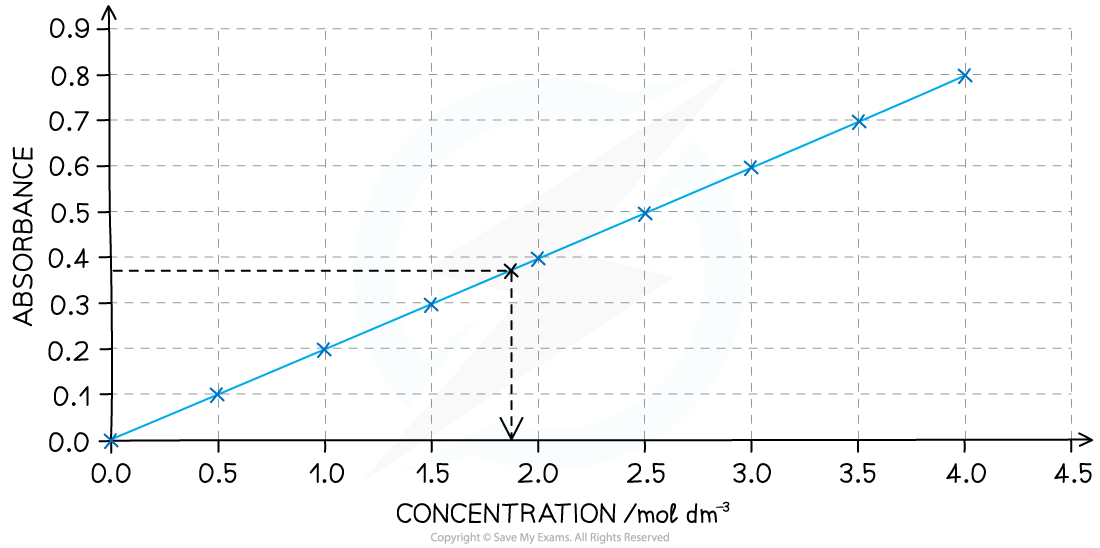
Calibration curve from colorimetry results, showing the absorbance of an unknown being used to determine the concentration

You've read 0 of your 5 free revision notes this week
Unlock more, it's free!
Did this page help you?
