Finding Ka (AQA A Level Chemistry) : Revision Note
Finding Ka
The Ka of a weak acid may be determined by finding the pH at the half equivalence point
At the half equivalence point Ka = [H+]

Key steps in the procedure
A pH probe or meter is calibrated before use
25 cm3 of 0.1 mol dm-3 ethanoic acid is pipetted into a conical flask and a few drops of phenolphthalein indicator are added
A burette is filled with 0.1 mol dm-3 sodium hydroxide solution
The contents of the conical flask are titrated against the sodium hydroxide until the indicator just turns pink
A further pipette containing 25 cm3 of the acid is then added to the flask and the pH is measured
Specimen Results
pH at half equivalence point = 4.75
Analysis
After the addition of the second portion of acid, the solution is effectively a half-neutralised sample of acid
This is the half-equivalence point at which the Ka = [H+]
By measuring the pH at that point you can convert it to [H+] and hence find the Ka of the acid
pH = 4.75
[H+] = 10-pH = 1.8 x 10-5 mol dm-3
∴ Ka = 1.8 x 10-5 mol dm-3
You can also say that the pKa = pH at the half-equivalence point
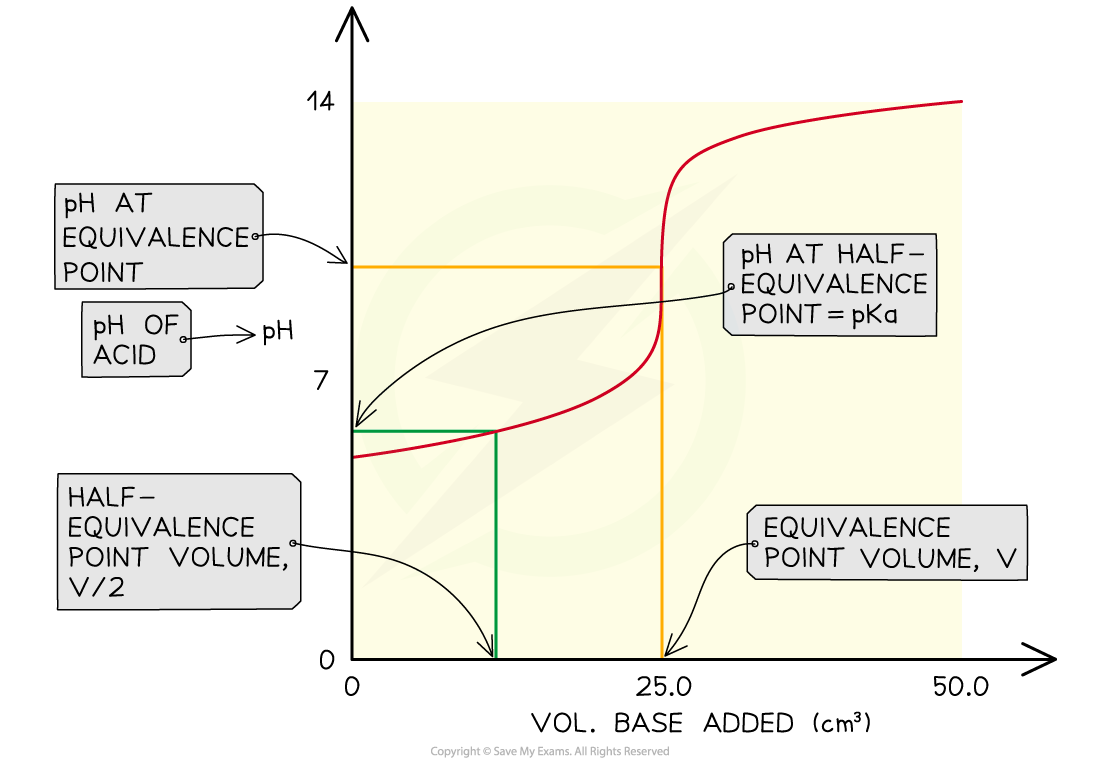
The pH at the half-equivalence point in a weak acid titration gives the pKa of the weak acid
Examiner Tips and Tricks
The sources of uncertainty in this experiment include the measurements made by the pipette and burette, and the judgement of the end-point of the titration.Be careful, there is a single uncertainty in the pipette measurements as one reading was taken but there is double the uncertainty for the burette reading as there was the initial and final readings.

You've read 0 of your 5 free revision notes this week
Unlock more, it's free!
Did this page help you?
