Entropy of Vaporisation (AQA A Level Chemistry): Revision Note
Exam code: 7405
Entropy of Vaporisation
The entropy change when water boils can be measured using a kettle and a top pan balance
At the boiling point, liquid water and water vapour exist in equilibrium so the free energy change is 0
Rearranging the Gibbs equation allows us to find the entropy change using the enthalpy change:
ΔGꝋ = ΔHꝋ – TΔSꝋ = 0
ΔHꝋ = TΔSꝋ
ΔSꝋ = ΔHꝋ / T
The sequence is:
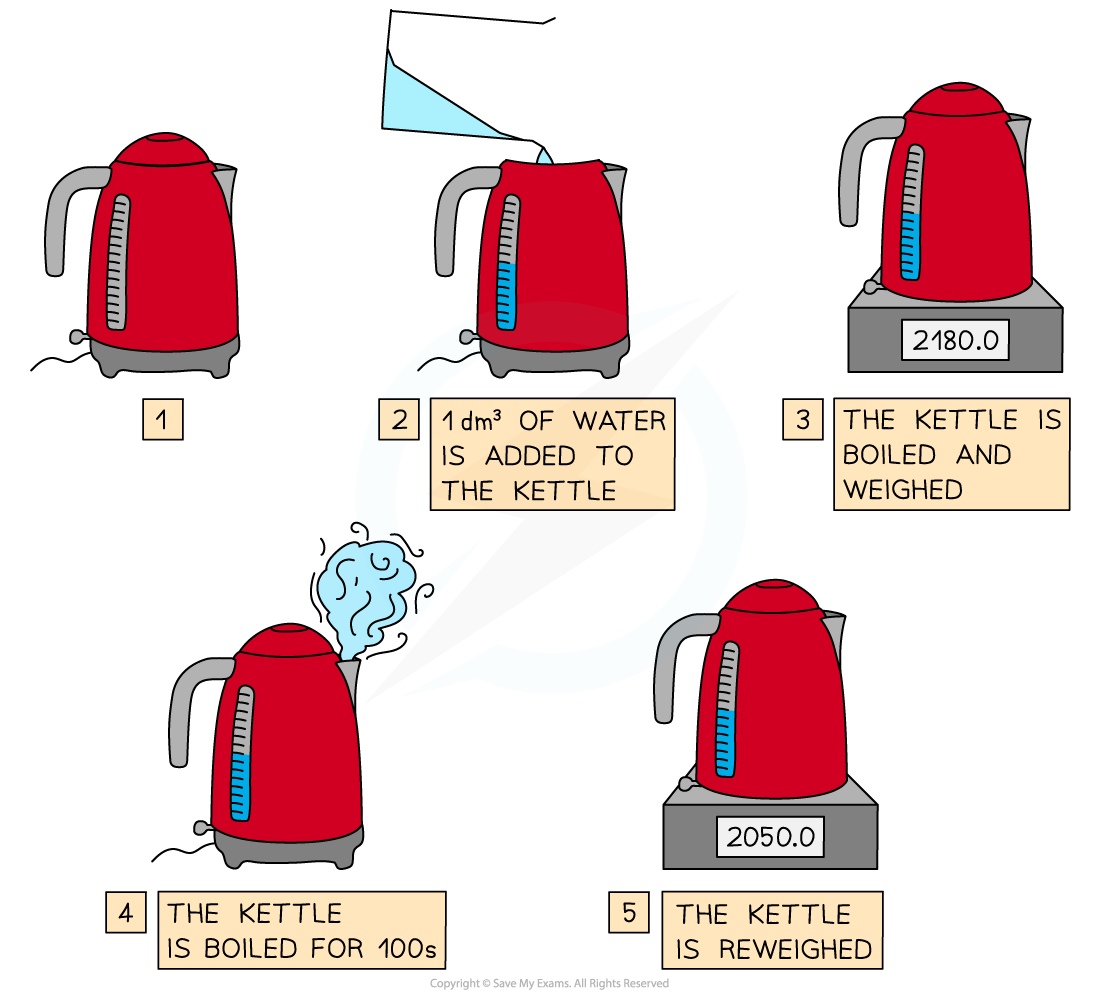
Determining the entropy of vaporisation by measuring the enthalpy change of boiling water
Steps in the procedure
A fixed volume of water is measured and added to an electric kettle - a suitable amount to use is 1 dm3
The power rating of the kettle must be known - it will usually be marked somewhere on the kettle
The water is boiled and then the kettle is switched off and weighed
The water is re-boiled by keeping the automatic cut-off switch depressed for 100 s
The kettle and contents are then re-weighed to find out how much water has been lost by evaporation
Practical tips
Make sure you have a balance that can read up to at least 2.5 kg
If you don't, you can weigh the water before and after the experiment in a lightweight container such as a plastic box or reduce the volume of water
The actual amount of water you use does not really matter as long as you can measure it and it covers the heating element in the kettle
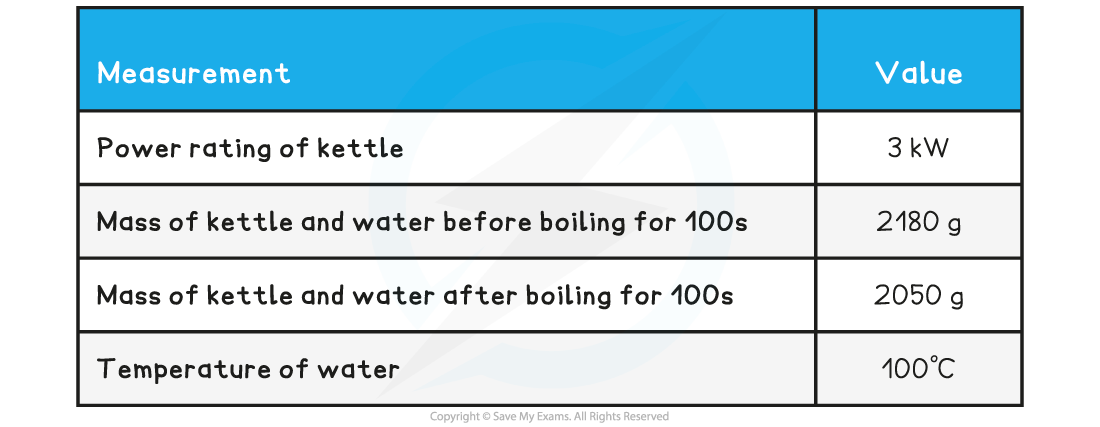
Specimen Results
Here is a set of typical results for this experiment
Specimen Results Table
Analysis
Household kettles have a typical power rating of 2 - 3 kW
Suppose you use a 3 kW kettle for this experiment; this means the kettle supplies 3 kJ of energy per second
If you boil the kettle for 100 s, then the kettle has supplied 300 kJ of energy to the water
Using the specimen results we can see that 130 g of water have boiled away, so the number of moles of water that evaporated are:
moles of water evaporated = 130 / 18 = 7.22 mol
The enthalpy change in kJ per mole of water is therefore:
enthalpy of vaporisation of water = 300 / 7.22 = 41.55 kJ mol-1
Changing this to J per mole gives:
enthalpy of vaporisation of water = 41.55 x 1000 = 41 550 J mol-1
The temperature in Kelvin at which water boils is:
temperature in Kelvin = 100 + 273 = 373 K
Substituting into the equation gives a value for the entropy of vaporisation of water
ΔSꝋ = ΔHꝋ / T
ΔSꝋ = 41 550 / 373 = 111.4 J mol-1

You've read 0 of your 5 free revision notes this week
Unlock more, it's free!
Did this page help you?