Formation of Coloured Ions (AQA A Level Chemistry): Revision Note
Exam code: 7405
Colour in Transition Metal Ions
Most transition element complexes are coloured
The transition metal ions can be identified by their colour

The large variety of coloured compounds is a defining characteristic of transition metals
A transition element complex solution which is coloured, absorbs wavelengths of light in the visible light region of the electromagnetic spectrum
The observed colour is the complementary colour which is made up of wavelengths of light that are transmitted or reflected
For example, copper(II) ions absorb light from the red end of the spectrum
The complementary colour observed is therefore blue-green (cyan)
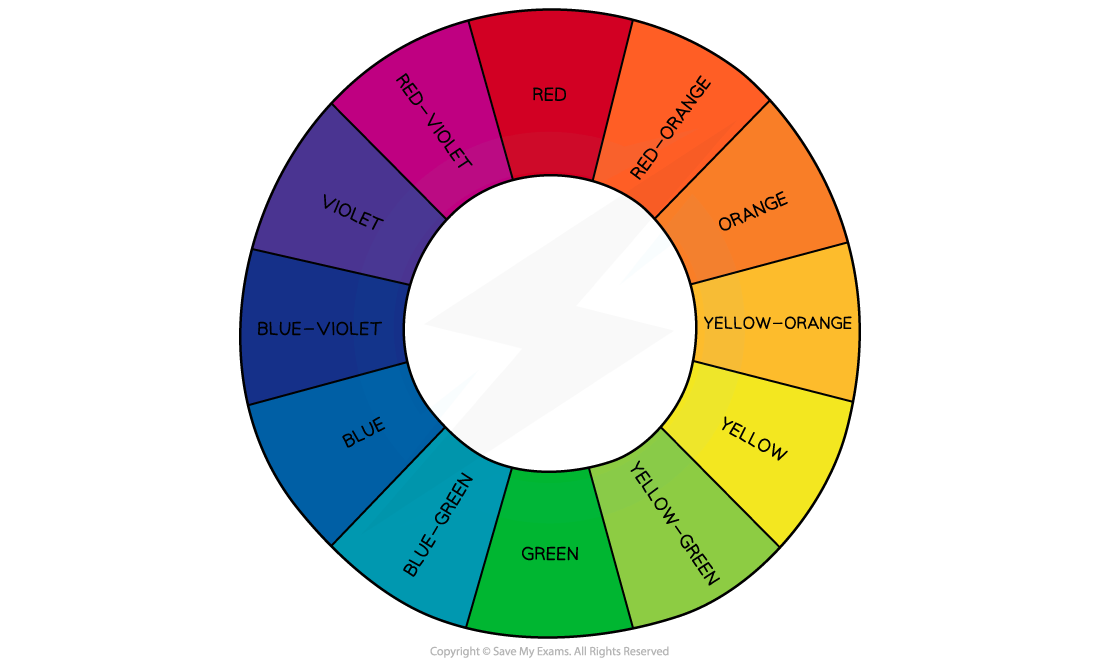
The colour wheel showing complementary colours in the visible light region of the electromagnetic spectrum
Electron promotion
In an isolated transition element ion (which is not bonded to any ligands), all of the 3d orbitals are equal in energy
The term used is degenerate
However, when ligands are attached to the central metal ion through dative covalent bonds, these orbitals are split into two sets of non-degenerate orbitals
The difference in energy between these two sets of orbitals is ΔE
When light shines on a solution containing a transition element complex, an electron will absorb this exact amount of energy (ΔE)
The amount of energy absorbed can be worked out by the equation:
ΔE = h x v
h = Planck's constant (6.626 x 10-34 m2 kg s-1)
v = frequency (Hertz, Hz or s-1)
The electron absorbs light energy that then excites it from a 3d orbital of lower energy to one of higher energy (excited state)
This is also called electron promotion
The other frequencies of light which are not absorbed combine to make the complementary colour
The diagram below shows an example of electron promotion in an octahedral complex of a nickel(II) Ni2+ ion
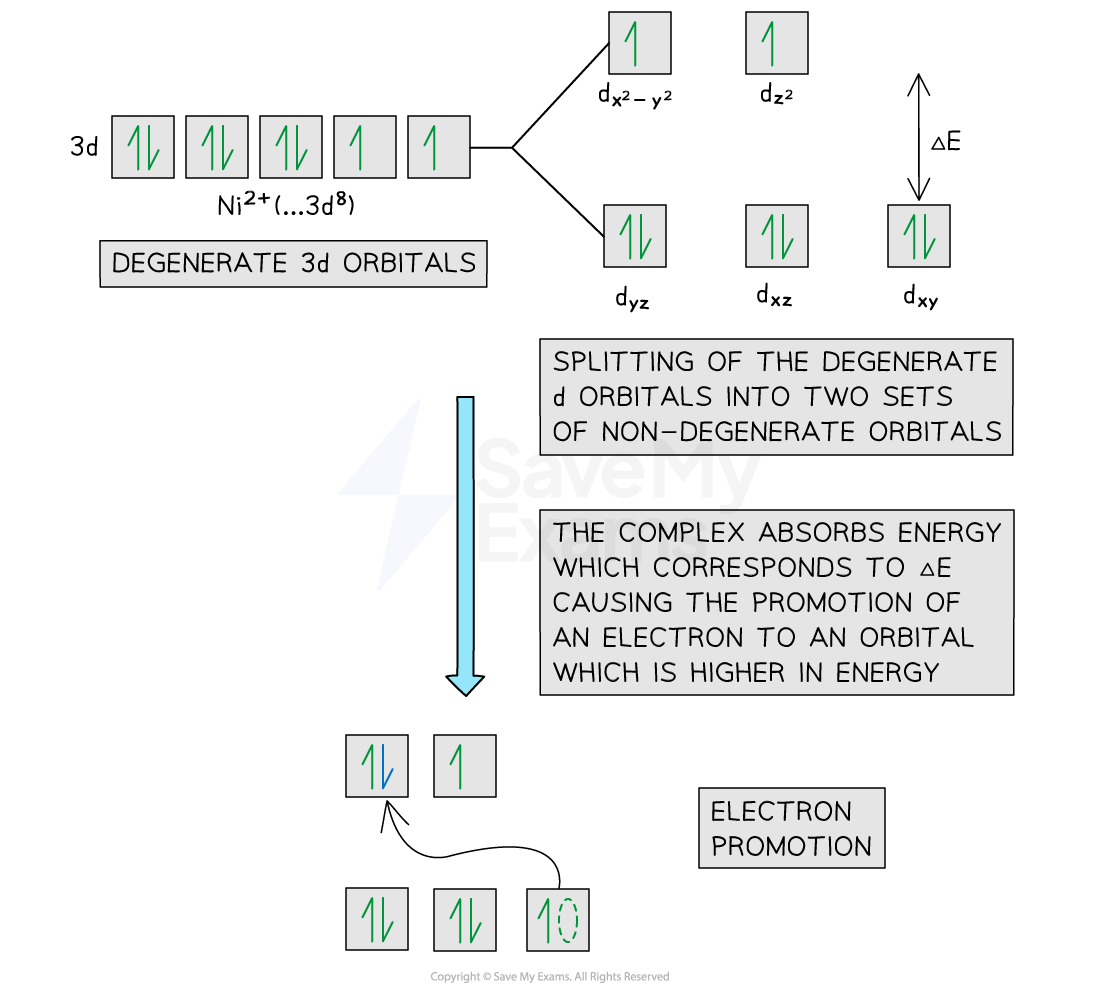
Electron promotion in a Ni(II) complex when light shines on the solution
Changes in Colour
Transition element complexes absorb the frequency of light which corresponds to the exact energy difference (ΔE) between their non-degenerate d orbitals
The frequencies of light which are not absorbed combine to make the complementary colour of the complex
It is the complementary colour which is transmitted and observed
However, the exact energy difference (ΔE) is affected by factors such as
the type of ligand
the coordination number
the oxidation state of the transition metal ion
Type of ligand
Different ligands will split the d orbital by a different amount of energy
This depends on the repulsion that the d orbital experiences from these ligands
Therefore, the size of ΔE and thus the frequency of light absorbed by the electrons will be slightly different
As a result, a different colour of light is absorbed by the complex solution and a different complementary colour is observed
This means that complexes with similar transition elements ions, but different ligands, can have different colours
For example, the [Cu(H2O)6]2+ complex has a light blue colour
Whereas the [Cu(NH3)4 (H2O)2]2+ has a dark blue colour
Despite the copper ion having an oxidation state of +2 in both complexes
This is evidence that the ligands surrounding the complex ion affect the colour of the complex
Coordination number
The coordination number also influences the strength of the metal ion-ligands interactions
Changing the coordination number generally involves changing the ligand as well, so it is a combination of these factors that alters the strength of the interactions
Oxidation State
The strength of the attraction between the transition metal ion and the electrons pairs in the dative covalent bonds can vary depending on the effective nuclear charge of the metal ion
Manganese(II) and iron(III) have the same electronic configuration, which is 1s22s22p63s23p63d5
[Mn(H2O)6]2+ appears pink because it absorbs in the green region of the spectrum, following the principle of complementary colours
The higher effective nuclear charge on aqueous [Fe(H2O)6]3+ means it has a stronger interaction with the ligands so it absorbs in the blue higher energy end of the spectrum and appears orange in colour
When the same metal is in a higher oxidation state that will also create a stronger interaction with the ligands
If you compare iron(II) and iron (III), [Fe(H2O)6]2+ absorbs in the red region and appears green, but [Fe(H2O)6]3+
absorbs in blue region and appears orange

The visible part of the electromagnetic spectrum shows the relationship between colour and energy. The red end of the spectrum is lower energy and the blue end is higher energy
Visible Light Spectroscopy
Spectroscopy is the study of the interactions between light and matter
A simple colorimeter is used to find the concentration of coloured transition metal ion solutions
The colorimeter passes different frequencies of light through a sample of the solution
The frequency absorbed is determined by the use of coloured filters and a detector
A filter is chosen matching the part of the spectrum where the absorption is strongest
This means choosing a filter that is the complementary colour to the colour of the solution
For example, a blue solution will absorb red, and therefore a red filter is used, so that only red light passes through the solution and maximum absorption occurs
Some of the light is absorbed by the solution and the rest passes through to the detector

The working principles of a visible light colorimeter
To determine the concentration of a coloured solution first a calibration curve has to be made
This involves measuring the absorption of a set of standard solutions whose concentration is known
The values are then plotted on a graph of absorption against concentration
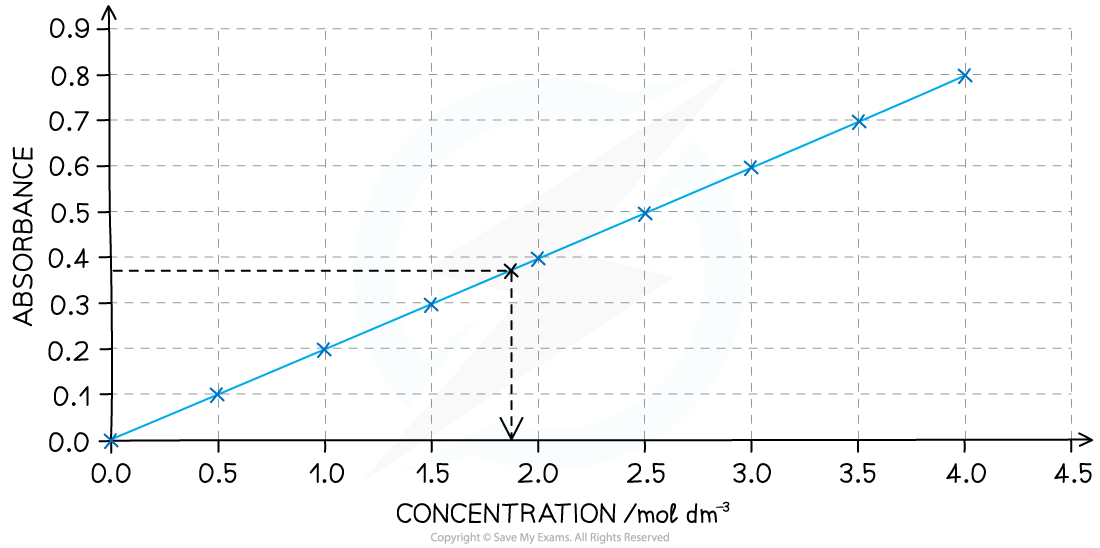
A calibration curve is used to find the concentration of unknown coloured solutions of transition metal ions
At lower concentrations the absorbance is directly proportional to the concentration of the coloured species so a straight line graph is obtained; this is known as the Beer-Lambert Law
Once the calibration curve is plotted then the absorbance of any unknown solution of the same complex ion can be measured
You can then deduce the concentration of the unknown sample by extrapolating from the absorbance to the calibration curve and down to the concentration
Limitations of visible light spectroscopy can be:
A very darkly coloured solution can be difficult for a colorimeter to read accurately
A very pale solution may also be at the limit of the colorimeters sensitivity; however a work around is to use ligand exchange to convert the complex into a more strongly coloured one
Adding thiocyanate ions to dilute solutions of [Fe(H2O)6]3+ produces a red coloured complex allowing the analyses of lower concentrations in iron(III
[Fe(H2O)6]3+ (aq) + SCN- (aq) ⇌ [Fe(H2O)5 SCN]2+ (aq) + H2O (l)
pale orange colourless blood-red
Examiner Tips and Tricks
You should know the factors that change the colour of transition metal complex ions and be able to write equations to give examples of those changes.

Unlock more, it's free!
Did this page help you?