Finding Activation Energy (AQA A Level Chemistry): Revision Note
Exam code: 7405
Finding Activation Energy
Finding the Activation Energy
Very often, the Arrhenius Equation is used to calculate the activation energy of a reaction
Either a question will give sufficient information for the Arrhenius equation to be used, or a graph can be plotted and the calculation done from the plot
Using the equation:
Remember, it is usually easier to use the version of the Arrhenius equation after natural logs of each side have been taken
Worked Example
Calculate the activation energy of a reaction which takes place at 400 K, where the rate constant of the reaction is 6.25 x 10-4 s-1.
A = 4.6 x 1013 and R = 8.31 J K-1 mol-1.
Answer:
Rearrange the equation for Ea:
Insert the values from the question:
Ea = 129095.85 J
Convert from J to kJ:
Ea = 129 kJ
Using an Arrhenius plot:
A graph of ln k against 1/T can be plotted, and then used to calculate Ea
This gives a line which follows the form y = mx + c
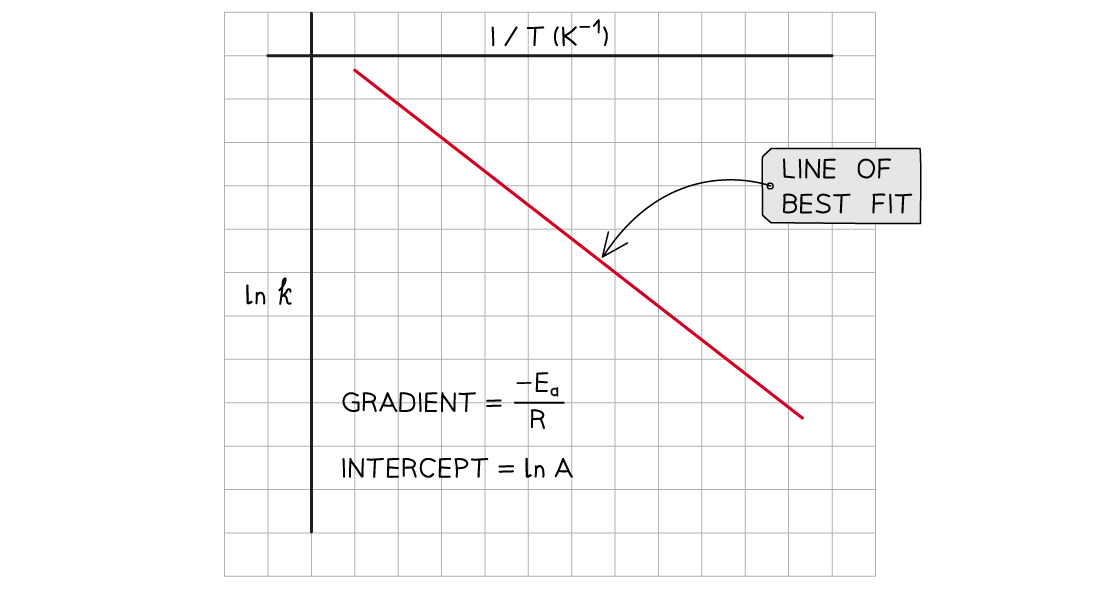
The graph of ln k against 1/T is a straight line with gradient -Ea/R
From the graph, the equation in the form of y = mx + c is:
Where:
y = ln k
x =
m =
(the gradient)
c = ln A (the y-intercept)
Worked Example
Complete the following table.
Temperature | 1/T | Time, t | Rate constant, k | ln k |
|---|---|---|---|---|
310 | 3.23 x 10-3 | 57 | -9.2 | |
335 | 31 | 3.01 x 10-4 | -8.1 | |
360 | 2.78 x 10-3 | 19 | 5.37 x 10-4 | -7.5 |
385 | 2.60 x 10-3 | 7 | 9.12 x 10-4 |
Plot a graph of ln k against 1/T.
Using the graph and following equation, calculate the activation energy, Ea, for this reaction.
Calculate the Arrhenius constant, A, for this reaction.
Answers:
The completed table is:
Temperature | 1/T | Time, t | Rate constant, k | ln k |
|---|---|---|---|---|
310 | 3.23 x 10-3 | 57 | 1.01 x 10-4 | -9.2 |
335 | 2.99 x 10-3 | 31 | 3.01 x 10-4 | -8.1 |
360 | 2.78 x 10-3 | 19 | 5.37 x 10-4 | -7.5 |
385 | 2.60 x 10-3 | 7 | 9.12 x 10-4 | -7.0 |
The graph of ln k against 1/T is:
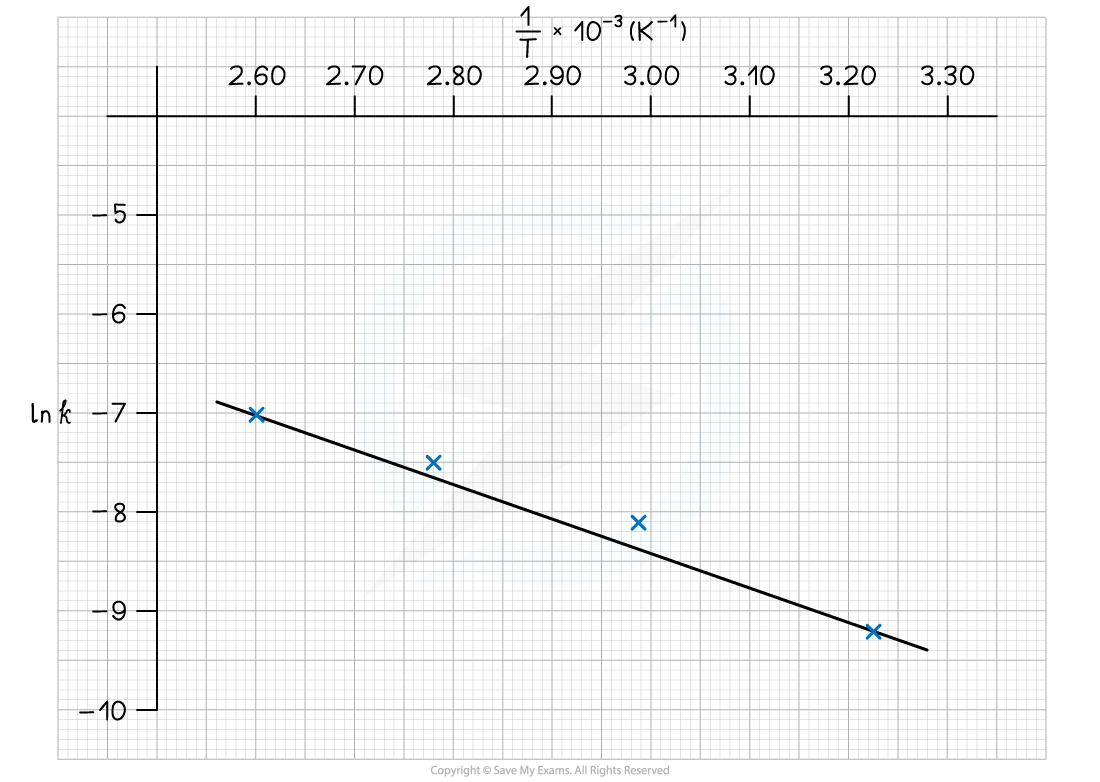
To calculate the activation energy, Ea:

Gradient = -Ea / R = -3666.6
Ea = -(-3666.6 x 8.31) = 30, 469 J mol-1
Ea = -(-3666.6 x 8.31) = 30.5 kJ mol-1
To calculate the Arrhenius constant, A:
Choose a point on the graph, e.g (2.60 x 10-3, -7.0)

Substitute values into the equation
-7.0 = (-3666.6 x 2.60 x 10-3) + ln A
-7.0 = -9.53 + ln A
ln A = 2.53
A =
A = 12.55
Examiner Tips and Tricks
You are not required to learn these equations. However, you do need to be able to rearrange them, and knowing them is helpful in understanding the effects of temperature on the rate constant.

Unlock more, it's free!
Did this page help you?