Comparing Lattice Enthalpies (AQA A Level Chemistry) : Revision Note
Comparing Lattice Enthalpies
How accurate are lattice enthalpies?
It is possible to calculate a theoretical value for the lattice enthalpy of an ionic solid. To do this you need to know
the geometry of the ionic solid
the charge on the ions
the distance between the ions
This has been calculated for a number of ionic solids and allows a comparison between theoretical lattice enthalpies and experimental lattice enthalpies obtained from Born-Haber cycles
Table comparing theoretical and experimental lattice enthalpies

You can see from the table that there is quite close agreement between the two values for the lattice enthalpy of sodium chloride
The calculation of the theoretical value is based on an assumption that the substance is a highly ionic compound with only electrostatic attraction between cations and anions
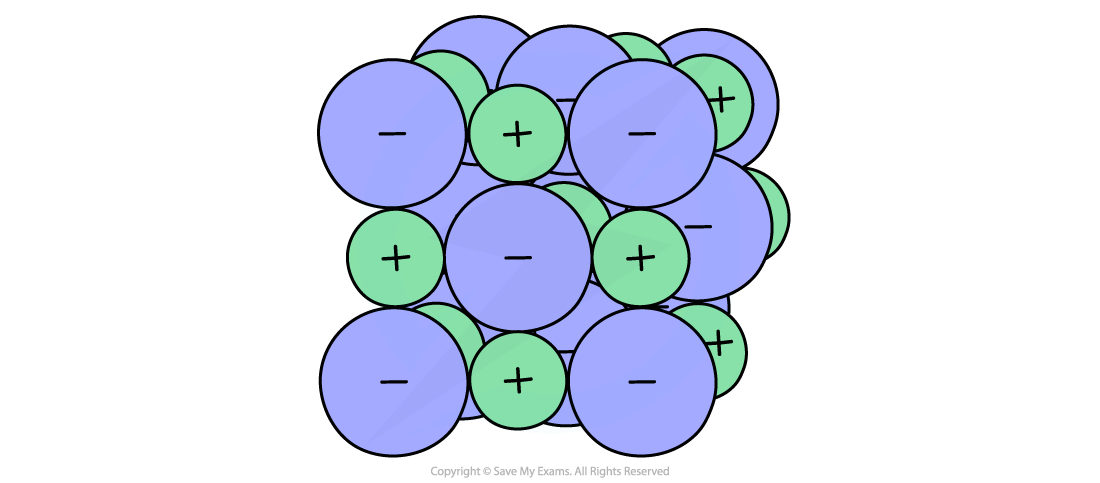
The ionic model for sodium chloride
However, the difference between theoretical and experimental lattice enthalpy increases for zinc sulfide
This suggests that the bonding is not purely ionic and some covalent character is present
This can be explained as follows:
Zinc is a smaller ion with a greater charge(+2) than sodium(+1)
Zinc ions attract electron density towards themselves, distorting the electron cloud and making the bonding slightly covalent
Sulfide ions are larger ions than chloride ions(-1) with a greater negative charge(-2)
The electron cloud around sulfide ions is more easily distorted than in chloride ions leading to further covalent character
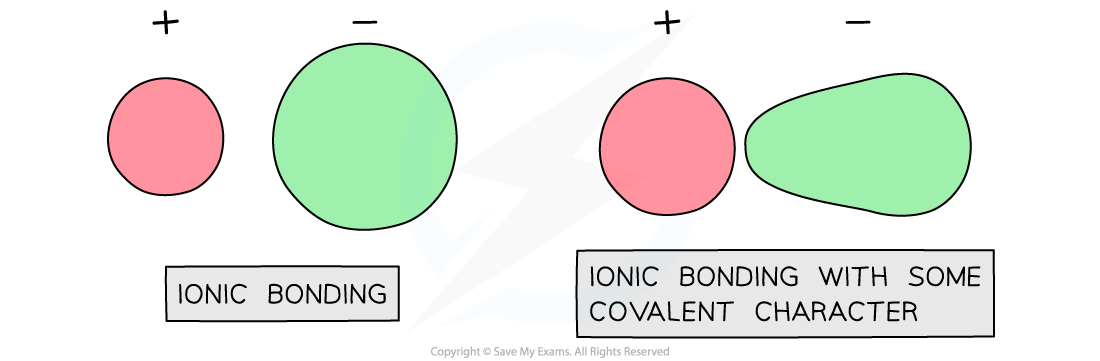
Covalent character in ionic compounds
As you move left to right across the period table the lattices become less ionic and more covalent leading to a discrepancy in the lattice enthalpy values
The result of these analyses provides strong evidence that supports the ionic model for some compounds like sodium chloride
Examiner Tips and Tricks
The distortion of the electron clouds is known as polarisation and illustrates that bonding is not either pure ionic or covalent, but rather a continuum between the two extremes.
Factors affecting lattice enthalpy
The two key factors which affect lattice energy, ΔHlattꝋ, are the charge and radius of the ions that make up the crystalline lattice
Ionic radius
The lattice energy becomes less exothermic as the ionic radius of the ions increases
This is because the charge on the ions is more spread out over the ion when the ions are larger
The ions are also further apart from each other in the lattice
The attraction between ions is between the centres of the ions involved, so the bigger the ions the bigger the distance between the centre of the ions
Therefore, the electrostatic forces of attraction between the oppositely charged ions in the lattice are weaker
For example, the lattice energy of caesium fluoride (CsF) is less exothermic than the lattice energy of potassium fluoride (KF)
Since both compounds contain a fluoride (F-) ion, the difference in lattice energy must be due to the caesium (Cs+) ion in CsF and potassium (K+) ion in KF
Potassium is a Group 1 and Period 4 element
Caesium is a Group 1 and Period 6 element
This means that the Cs+ ion is larger than the K+ ion
There are weaker electrostatic forces of attraction between the Cs+ and F- ions compared to K+ and F- ions
As a result, the lattice energy of CsF is less exothermic than that of KF

The lattice energies get less exothermic as the ionic radius of the ions increases
Ionic charge
The lattice energy gets more exothermic as the ionic charge of the ions increases
The greater the ionic charge, the higher the charge density
This results in stronger electrostatic attraction between the oppositely charged ions in the lattice
As a result, the lattice energy is more exothermic
For example, the lattice energy of calcium oxide (CaO) is more exothermic than the lattice energy of potassium chloride (KCl)
Calcium oxide is an ionic compound which consists of calcium (Ca2+) and oxide (O2-) ions
Potassium chloride is formed from potassium (K+) and chloride (Cl-) ions
The ions in calcium oxide have a greater ionic charge than the ions in potassium chloride
This means that the electrostatic forces of attraction are stronger between the Ca2+ and O2- compared to the forces between K+ and Cl-
Therefore, the lattice energy of calcium oxide is more exothermic, as more energy is released upon its formation from its gaseous ions
Ca2+ and O2- are also smaller ions than K+ and Cl-, so this also adds to the value for the lattice energy being more exothermic

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
