Testing for Organic Functional Groups (AQA A Level Chemistry): Revision Note
Exam code: 7405
Testing for Organic Functional Groups
REQUIRED PRACTICAL 6
For this practical, you need to be able to give the tests and positive results for the following functional groups:
Alkenes
Alcohols
Aldehydes
Carboxylic Acids
Testing for an Alkene
Halogens can be used to test if a molecule is unsaturated (i.e. contains a double bond)
Br2(aq) is an orange-yellow solution, called bromine water
The unknown compound is shaken with the bromine water
If the compound is unsaturated, an addition reaction will take place and the coloured solution will decolourise

The bromine water test is the standard test for unsaturation in alkenes
Testing an Alcohol
Alcohols can be classified as either primary, secondary or tertiary, depending on the placement of the -OH group
Primary and secondary alcohols can both be oxidised, but tertiary alcohols cannot
To test for the alcohol functional group, add a small amount (1 cm3) of the substance to a test tube using a pipette
Then, add a small amount (1 cm3) of a suitable oxidising agent to the sample using a different pipette
The most commonly used oxidising agent for this test is acidified potassium dichromate solution (K2Cr2O7, acidified with H2SO4)
Add a stopper to the test tube and shake well
Place in a hot water bath (heated to around 60 oC) for a few minutes
If a primary or secondary alcohol are present, then the colour will change from orange to green
If a tertiary alcohol is present, then nothing will happen - the solution will remain orange
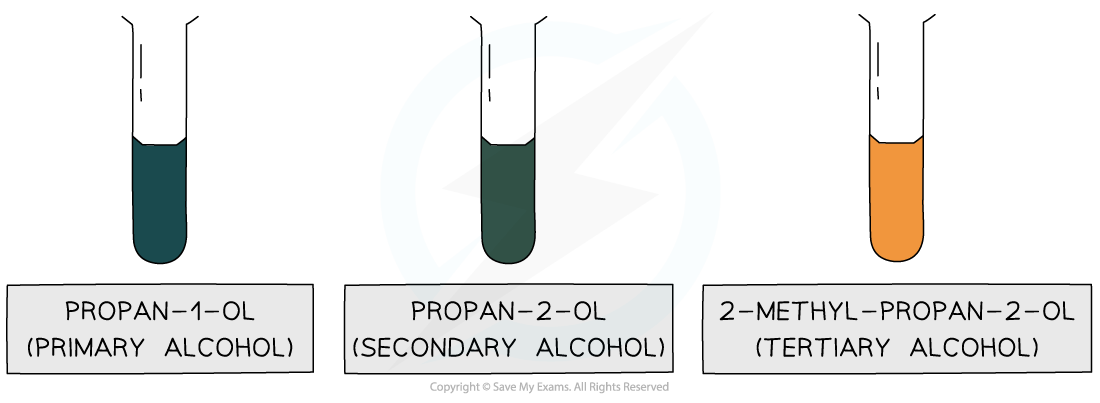
Positive test results of the oxidation of a primary, secondary and tertiary alcohol
Testing for an Aldehyde
Fehling’s solution
Fehling’s solution is an alkaline solution containing copper(II) ions which act as the oxidising agent
When warmed with an aldehyde, the aldehyde is oxidised to a carboxylic acid and the Cu2+ ions are reduced to Cu+ ions
In the alkaline conditions, the carboxylic acid formed will be neutralised to a carboxylate ion (the -COOH will lose a proton to become -COO– )
The carboxylate ion (-COO–) will form a salt with a positively charged metal ion such as sodium (-COO–Na+)
The clear blue solution turns opaque due to the formation of a red precipitate, copper(I) oxide
Ketones cannot be oxidised and therefore give a negative test when warmed with Fehling’s solution

The copper(II) ions in Fehling’s solution are oxidising agents, oxidising the aldehyde to a carboxylic acid and getting reduced themselves to copper(I) ions in the Cu2O precipitate
Tollens’ reagent
Tollens' reagent is an aqueous alkaline solution of silver nitrate in excess ammonia solution
Tollens' reagent is also called ammoniacal silver nitrate solution
When warmed with an aldehyde, the aldehyde is oxidised to a carboxylic acid and the Ag+ ions are reduced to Ag atoms
In the alkaline conditions, the carboxylic acid will become a carboxylate ion and form a salt
The Ag atoms form a silver ‘mirror’ on the inside of the tube
Ketones cannot be oxidised and therefore give a negative test when warmed with Tollens’ reagent

The Ag+ ions in Tollens’ reagent are oxidising agents, oxidising the aldehyde to a carboxylic acid and getting reduced themselves to silver atoms
Testing for a Carboxylic Acid
Carboxylic acids in solution have a pH of around 3, so measuring the pH is a way of testing for the presence of the carboxylic acid functional group in an organic sample
The end of a glass rod could be dipped into the solution and then carefully dripped onto indicator paper
Or, a pH probe could be used, which would give you an exact pH
Since carboxylic acids are acids, they will react with a carbonate solution to produce carbon dioxide gas
1-2 cm3 of sodium carbonate (Na2CO3) or sodium hydrogen carbonate solution (NaHCO3) could be added using a pipette
If bubbles of gas are seen, this is a good indicator that the solution is a carboxylic acid
If an exam question asks you to simply distinguish between different types of organic compound, and the carboxylic acid is the only organic compound present which would react in this way with a carbonate solution, then this is enough
The gas produced could then be bubbled into limewater
If the limewater turns milky or cloudy, then this proves that the gas produced was carbon dioxide


Unlock more, it's free!
Did this page help you?