Classifying Alcohols (AQA A Level Chemistry) : Revision Note
Classification of Alcohols
Primary alcohols are alcohols in which the carbon atom bonded to the -OH group is attached to one other carbon atom (or alkyl group)
Secondary alcohols are alcohols in which the carbon atom bonded to the -OH group is attached to two other carbon atoms (or alkyl groups)
Tertiary alcohols are alcohols in which the carbon atom bonded to the -OH group is attached to three other carbon atoms (or alkyl groups)

Classifying primary, secondary and tertiary alcohols and alcohols with more than one alcohol group
Only primary and secondary alcohols can get oxidised when mildly oxidised with acidified K2Cr2O7
Primary alcohols get mildly oxidised to aldehydes
Secondary alcohols get mildly oxidised to ketones
Tertiary alcohols do not undergo oxidation with acidified K2Cr2O7
Therefore, only the oxidation of primary and secondary alcohols will change the colour of K2Cr2O7 solution as the orange Cr2O72- ions are reduced to green Cr3+ ions
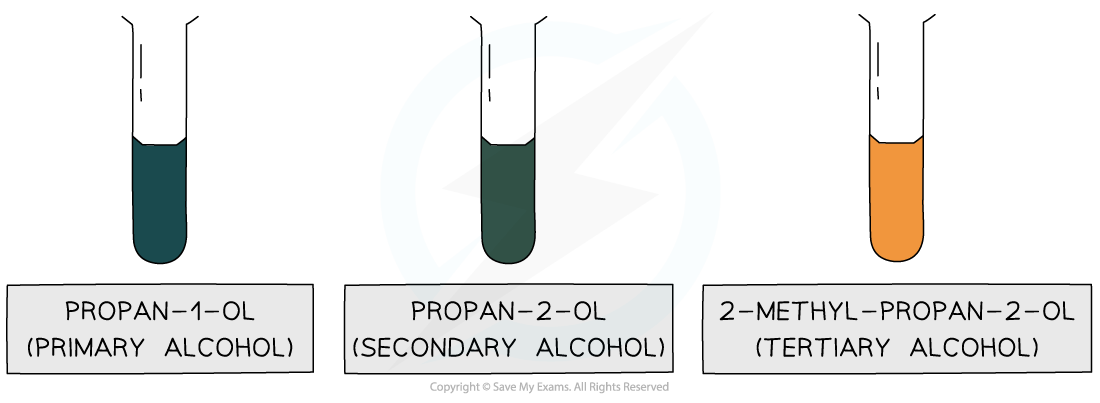
Only primary and secondary alcohols can be oxidised, turning the orange solution green
No colour change is observed with a tertiary alcohol

You've read 0 of your 5 free revision notes this week
Unlock more, it's free!
Did this page help you?
