Addition Polymers (AQA A Level Chemistry) : Revision Note
Addition Polymers
Addition polymerisation
Addition polymerisation is one of the most important addition reactions of alkenes which form the basis of the plastics industry
Addition polymerisation is the reaction in which many monomers containing at least one C=C double bond form long chains of polymers as the only product
Just like in other addition reactions of alkenes, the π-bond in each C-C bond breaks and then the monomers link together to form new C-C single bonds
A polymer is a long-chain molecule that is made up of many repeating units
The small, reactive molecules that join together to form the polymer are called monomers
A polymerisation reaction can be represented by a general formula or by using displayed formulae
Eg. poly(ethene) and poly(chloroethene) (also known as PVC) are polymers made up of the ethene and chloroethene monomers respectively and are commonly used in making plastics

The general formulae of the addition polymerisation of ethene (1) and chloroethene (2)
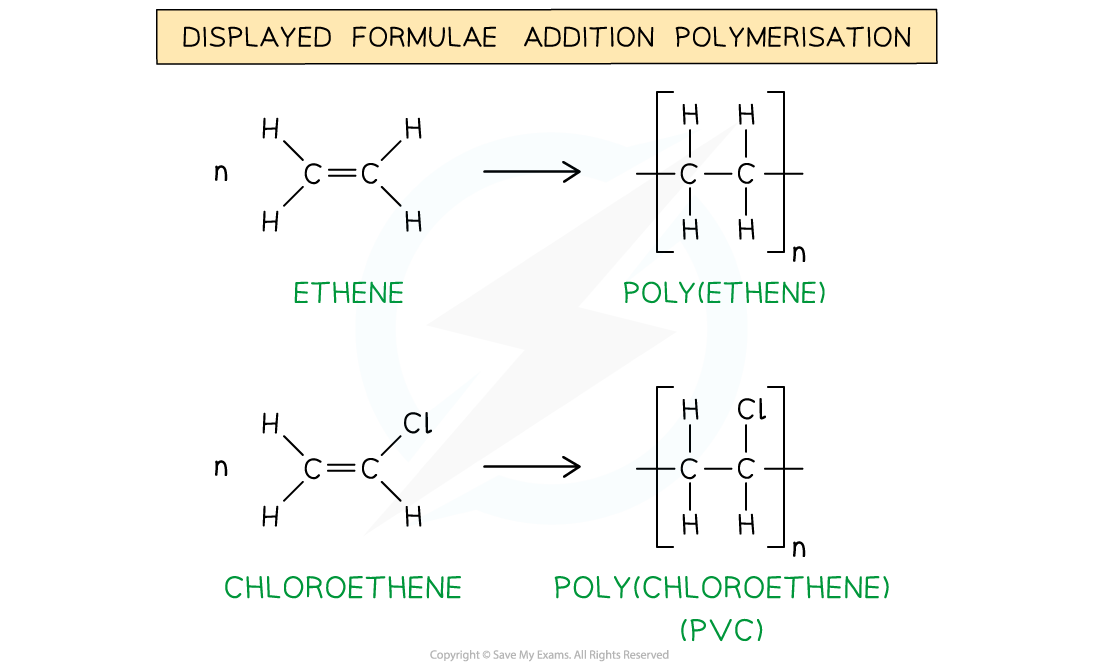
The displayed formulae of the addition polymerisation of ethene (1) and chloroethene (2)
Just like any other addition reaction of alkenes, addition polymerisation gives only one product
Plasticisers
The properties of PVC can be altered by adding a plasticiser
PVC is rigid enough to be used for making drainpipes
With a plasticiser PVC can be made flexible enough to make pool liners
A plasticiser makes a polymer more flexible by preventing the polymer chains from being close to one another
This disrupts the van der Waals forces between the chains, making them weaker
As a result, the chains slide over each other as easily

Deducing repeat units
A repeat unit is the smallest group of atoms that when connected one after the other make up the polymer chain
It is represented by square brackets in the displayed and general formula
In poly(alkenes) (such as poly(ethene)) and substituted poly(alkenes) (such as PVC) made of one type of monomer the repeating unit is the same as the monomer except that the C=C double bond is changed to a C-C single bond

The repeating units of poly(ethene) and poly(chloroethene) are similar to their monomer except that the C=C bond has changed into a C-C bond
Worked Example
Identifying monomersIdentify the monomers present in the given sections of addition polymer molecules:
Answer 1:
When ethenol, CH(OH)=CH2, is polymerised, the C=C double bond opens to produce a repeating unit of -CH(OH)-CH2- This gives the polymer poly(ethenol)

Answer 2:
To find the monomer, first the repeating unit should be deduced. Repeating units have only 2 carbons in the polymer main chain

Since the repeating unit is now found, it can be concluded that the monomer is prop-2-enoic acid

Answer 3:
Again, the repeating unit only has 2 carbons in the polymer chain which in this case are two carbon atoms that each contain one OH group
Thus, when ethene-1,2-diol, CH(OH)=CH(OH), is polymerised, the C=C double bond opens to produce a repeating unit of -CH(OH)-CH(OH)- which gives the polymer poly(ethene-1,2-diol)

Examiner Tips and Tricks
The section of the polymer chain shown inside the square brackets by the structural or displayed formula is the repeat unit and not the monomerThe monomer is the same as the repeat unit except for that it has C=C bonds instead of C-C bonds

You've read 0 of your 5 free revision notes this week
Unlock more, it's free!
Did this page help you?
