Classification of an Element (AQA A Level Chemistry) : Revision Note
The Periodic Table: Structure & Classification
The periodic table is a list of all known elements arranged in order of increasing atomic number, from 1 to 118.
In addition to this, the elements are arranged in such a way that atoms with the same number of shells are placed together, and atoms with similar electronic configurations in the outer shell are also placed together. This is achieved as follows:
The elements are arranged in rows and columns.
Elements with one shell are placed in the first row (i.e. H and He)
Elements with two shells are placed in the second row (Li to Ne) and so on.
A row of elements thus arranged is called a period. The period number, n, is the outer energy level that is occupied by electrons.
In addition, the elements are aligned vertically (in columns) with other elements in different rows, if they share the same outer-shell electronic configuration
The outer electrons are known as the valence electrons.
A column of elements thus arranged is called a group
The Periodic Table
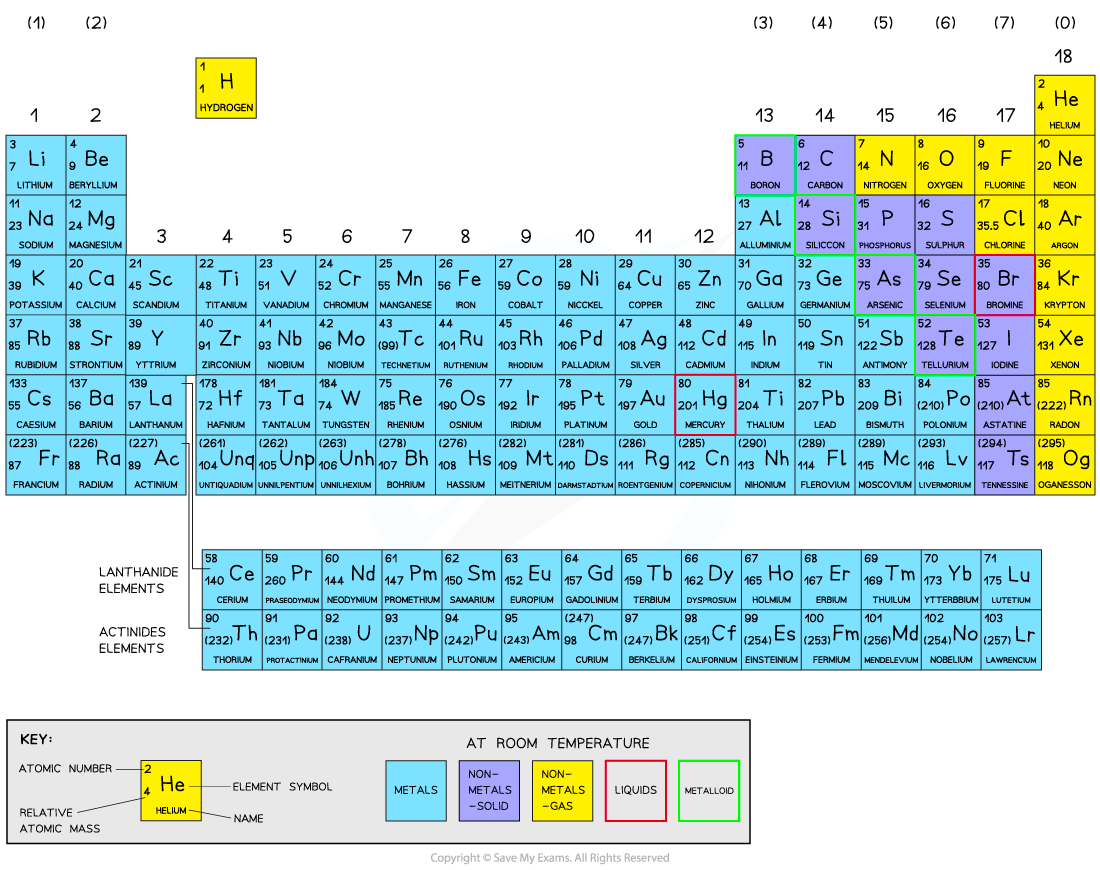
Since the electronic configurations of H and He are unusual, they do not fit comfortably into any group. They are thus allocated a group based on similarities in physical and chemical properties with other members of the group
He is placed in group 0 on this basis, but hydrogen does not behave like any other element and so is placed in a group of its own

The blocks of the periodic table
All elements belong to one of four main blocks: the s-block, the p-block, the d-block and the f-block
The s-block elements are all those with only s electrons in the outer shell
The p-block elements are all those with at least one p-electron in the outer shell
The d-block elements are all those with at least one d-electron and at least one s-electron but no f or p electrons in the outer shell (up to 5d)
The f-block elements are all those with at least one f-electron and at least one s-electron but no d or p electrons in the outer shell
The physical and chemical properties of elements in the periodic table show clear patterns related to the position of each element in the table
Elements in the same group show similar properties, and properties change gradually as you go across a period
As atomic number increases, the properties of the elements show trends which repeat themselves in each period of the periodic table
These trends are known as periodic trends and the study of these trends in known as periodicity
Reactions of Period 3 Elements
Reactions with oxygen
The reactions of period 3 elements with oxygen can be summarised as follows:

Reactions with chlorine
The reactions of period 3 elements with chlorine can be summarised as follows:

Reaction of sodium & magnesium with water
Sodium reacts vigorously with cold water:
2Na (s) + 2H2O (l) → 2NaOH (aq) + H2 (g)
The sodium melts into a ball and moves across the water surface until it disappears
Hydrogen gas is given off
The solution formed is strongly alkaline (pH 14) due to the sodium hydroxide which is formed

The diagram shows the reaction of sodium with cold water
Magnesium reacts extremely slowly with cold water:
Mg (s) + 2H2O (l) → Mg(OH)2 (aq) + H2 (g)
The solution formed is weakly alkaline (pH 9-10) as magnesium hydroxide is only slightly soluble
However, when magnesium is heated in steam, it reacts vigorously with steam to make magnesium oxide and hydrogen gas:
Mg (s) + H2O (g) → MgO (s) + H2 (g)

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
