The Role of Haemoglobin (OCR A Level Biology): Revision Note
Exam code: H420
The Role of Haemoglobin
Transport of oxygen
The majority of oxygen transported around the body is bound to the protein haemoglobin in red blood cells
Red blood cells are also known as erythrocytes
Each molecule of haemoglobin contains four haem groups, each able to bond with one molecule of oxygen
This means that each molecule of haemoglobin can carry four oxygen molecules, or eight oxygen atoms in total
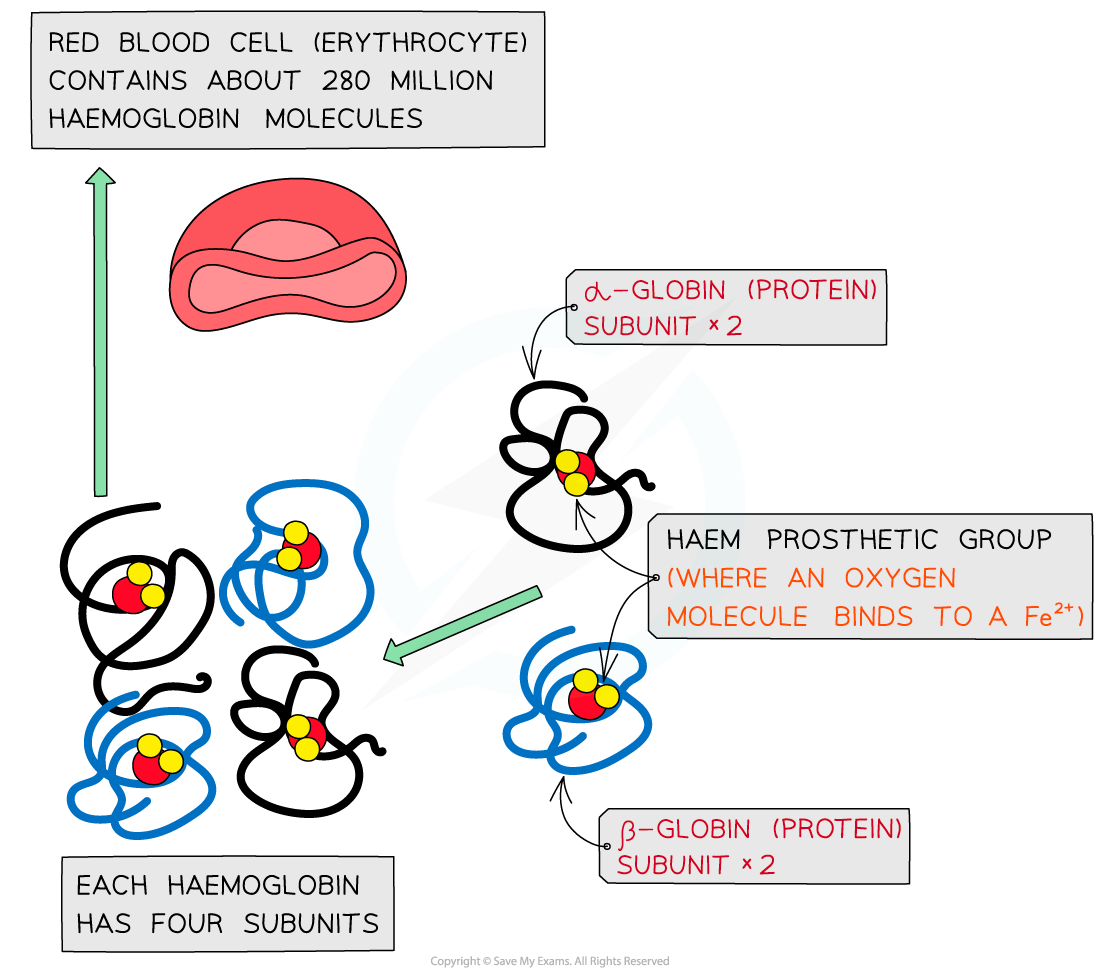
Haemoglobin proteins are made up of four subunits, each of which contains a region called a haem group to which oxygen can bind
When oxygen binds to haemoglobin, oxyhaemoglobin is formed
Oxygen + Haemoglobin Oxyhaemoglobin
4O2 + Hb Hb4O 2
The binding of the first oxygen molecule results in a conformational change in the structure of the haemoglobin molecule, making it easier for each successive oxygen molecule to bind; this is cooperative binding
The reverse of this process happens when oxygen dissociates in the tissues
Carbon dioxide transport
Waste carbon dioxide produced during respiration diffuses from the tissues into the blood
There are three main ways in which carbon dioxide is transported around the body
A very small percentage of carbon dioxide dissolves directly in the blood plasma and is transported in solution
Carbon dioxide can bind to haemoglobin, forming carbaminohaemoglobin
A much larger percentage of carbon dioxide is transported in the form of hydrogen carbonate ions (HCO3-)
Formation of hydrogen carbonate ions
Carbon dioxide diffuses from the plasma into red blood cells
Inside red blood cells carbon dioxide combines with water to form H2CO3
CO2 + H2O ⇌ H2CO3
Red blood cells contain the enzyme carbonic anhydrase which catalyses the reaction between carbon dioxide and water
Without carbonic anhydrase this reaction proceeds very slowly
The plasma contains very little carbonic anhydrase hence H2CO3 forms more slowly in plasma than in the cytoplasm of red blood cells
Carbonic acid dissociates readily into H+ and HCO3- ions
H2CO3 ⇌ HCO3– + H+
Hydrogen ions can combine with haemoglobin, forming haemoglobinic acid and preventing the H+ ions from lowering the pH of the red blood cell
Haemoglobin is said to act as a buffer in this situation
The hydrogen carbonate ions diffuse out of the red blood cell into the blood plasma where they are transported in solution

Carbon dioxide can be transported in the form of hydrogen carbonate ions
The Chloride shift
The chloride shift is the movement of chloride ions into red blood cells that occurs when hydrogen carbonate ions are formed
Hydrogen carbonate ions are formed by the following process
Carbon dioxide diffuses into red blood cells
The enzyme carbonic anhydrase catalyses the combining of carbon dioxide and water to form carbonic acid (H2CO3)
CO2 + H2O ⇌ H2CO3
Carbonic acid dissociates to form hydrogen carbonate ions and hydrogen ions
H2CO3 ⇌ HCO3- + H+
Negatively charged hydrogen carbonate ions formed from the dissociation of carbonic acid are transported out of red blood cells via a transport protein in the membrane
To prevent an electrical imbalance, negatively charged chloride ions are transported into the red blood cells via the same transport protein
If this did not occur then red blood cells would become positively charged as a result of a buildup of hydrogen ions formed from the dissociation of carbonic acid
Examiner Tips and Tricks
Be sure to learn the differences between the Bohr shift and chloride shift; the Bohr shift occurs when a high partial pressure of carbon dioxide causes haemoglobin to release oxygen into respiring tissues while the chloride shift is the movement of chloride ions into red blood cells.

Unlock more, it's free!
Did this page help you?