Enzyme Action (OCR A Level Biology) : Revision Note
Mechanism of Enzyme Action
Enzymes have an active site where specific substrates bind forming an enzyme-substrate complex
The active site of an enzyme has a specific shape to fit a specific substrate
Extremes of heat or pH can change the shape of the active site, preventing substrate binding – this is called denaturation (the enzyme is said to be denatured)
Substrates collide with the enzymes active site and this must happen at the correct orientation and speed in order for a reaction to occur

The active site of an enzyme has a specific shape to fit a specific substrate (when the substrate binds an enzyme-substrate complex is formed)
Enzyme specificity
The specificity of an enzyme is a result of the complementary nature between the shape of the active site on the enzyme and its substrate(s)
The shape of the active site (and therefore the specificity of the enzyme) is determined by the complex tertiary structure of the protein that makes up the enzyme:
Proteins are formed from chains of amino acids held together by peptide bonds
The order of amino acids determines the shape of an enzyme
If the order is altered, the resulting three-dimensional shape changes

An example of enzyme specificity – the enzyme catalase can bind to its substrate hydrogen peroxide as they are complementary in shape, whereas DNA polymerase is not
The enzyme-substrate complex
An enzyme-substrate complex forms when an enzyme and its substrate join together
The enzyme-substrate complex is only formed temporarily before the enzyme catalyses the reaction and the product(s) are released

The temporary formation of an enzyme-substrate complex
The lock-and-key hypothesis
Enzymes are globular proteins
This means their shape (as well as the shape of the active site of an enzyme) is determined by the complex tertiary structure of the protein that makes up the enzyme and is therefore highly specific
In the 1890’s the first model of enzyme activity was described by Emil Fischer:
He suggested that both enzymes and substrates were rigid structures that locked into each other very precisely, much like a key going into a lock
This is known as the ‘lock-and-key hypothesis’

The lock-and-key hypothesis
The induced-fit hypothesis
The lock-and-key model was later modified and adapted to our current understanding of enzyme activity, permitted by advances in techniques in the molecular sciences
The modified model of enzyme activity (first proposed in 1959) is known as the ‘induced-fit hypothesis’
Although it is very similar to the lock and key hypothesis, in this model the enzyme and substrate interact with each other:
The enzyme and its active site (and sometimes the substrate) can change shape slightly as the substrate molecule enters the enzyme
These changes in shape are known as conformational changes
The conformational changes ensure an ideal binding arrangement between the enzyme and substrate is achieved
This maximises the ability of the enzyme to catalyse the reaction
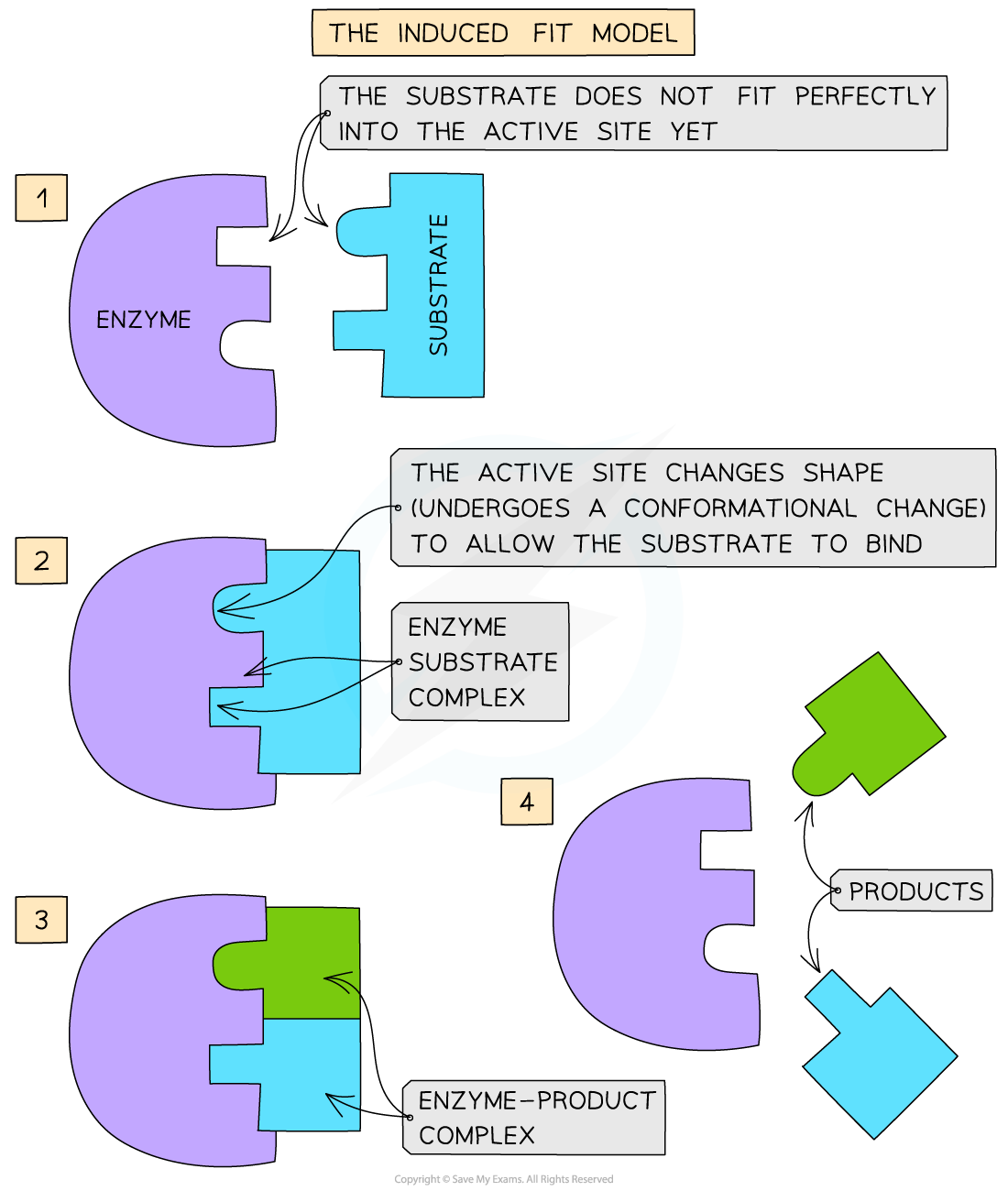
The induced-fit hypothesis
Enzymes and the lowering of activation energy
All chemical reactions are associated with energy changes
For a reaction to proceed there must be enough activation energy
Activation energy is the amount of energy needed by the substrate to become just unstable enough for a reaction to occur and for products to be formed
Enzymes speed up chemical reactions because they reduce the stability of bonds in the reactants
The destabilisation of bonds in the substrate makes it more reactive
Rather than lowering the overall energy change of the reaction, enzymes work by providing an alternative energy pathway with a lower activation energy
Without enzymes, extremely high temperatures or pressures would be needed to reach the activation energy for many biological reactions
Enzymes avoid the need for these extreme conditions (that would otherwise kill cells)

The activation energy of a chemical reaction is lowered by the presence of a catalyst (i.e. an enzyme)

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
