Globular Proteins (OCR A Level Biology) : Revision Note
Globular Proteins
Globular
Globular proteins are compact, roughly spherical (circular) in shape and soluble in water
Globular proteins form a spherical shape when folding into their tertiary structure because:
their non-polar hydrophobic R groups are orientated towards the centre of the protein away from the aqueous surroundings and
their polar hydrophilic R groups orientate themselves on the outside of the protein
This orientation enables globular proteins to be (generally) soluble in water as the water molecules can surround the polar hydrophilic R groups
The solubility of globular proteins in water means they play important physiological roles as they can be easily transported around organisms and be involved in metabolic reactions
The folding of the protein due to the interactions between the R groups results in globular proteins having specific shapes. This also enables globular proteins to play physiological roles, for example, enzymes can catalyse specific reactions and immunoglobulins (antibodies) can respond to specific antigens
Some globular proteins are conjugated proteins that contain a prosthetic group eg. haemoglobin which contains the prosthetic group called haem
Haemoglobin
Haemoglobin is a globular protein which is an oxygen-carrying pigment found in vast quantities in red blood cells
It has a quaternary structure as there are four polypeptide chains. These chains or subunits are globin proteins (two α–globins and two β–globins) and each subunit has a prosthetic haem group
The four globin subunits are held together by disulphide bonds and arranged so that their hydrophobic R groups are facing inwards (helping preserve the three-dimensional spherical shape) and the hydrophilic R groups are facing outwards (helping maintain its solubility)
The arrangements of the R groups is important to the functioning of haemoglobin. If changes occur to the sequence of amino acids in the subunits this can result in the properties of haemoglobin changing. This is what happens to cause sickle cell anaemia (where base substitution results in the amino acid valine (non-polar) replacing glutamic acid (polar) making haemoglobin less soluble)
The prosthetic haem group contains an iron II ion (Fe2+) which is able to reversibly combine with an oxygen molecule forming oxyhaemoglobin and results in the haemoglobin appearing bright red
Each haemoglobin with the four haem groups can therefore carry four oxygen molecules (eight oxygen atoms)
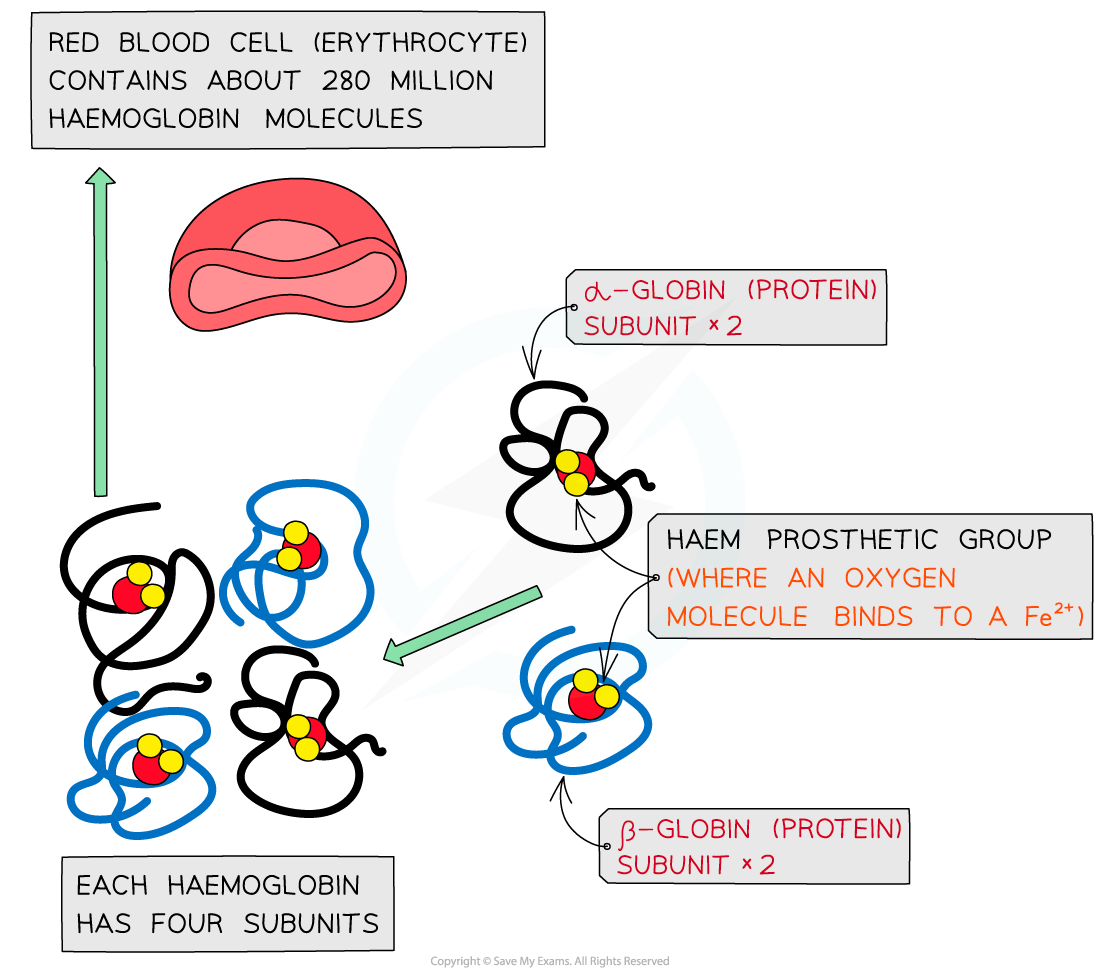
The structure of haemoglobin showing the α–globin and β–globin subunits, the prosthetic haem group with oxygen molecules bonded to form oxyhaemoglobin
Haemoglobin is responsible for binding oxygen in the lung and transporting the oxygen to tissue to be used in aerobic metabolic pathways
As oxygen is not very soluble in water and haemoglobin is, oxygen can be carried more efficiently around the body when bound to the haemoglobin
The presence of the haem group (and Fe2+) enables small molecules like oxygen to be bound more easily because as each oxygen molecule binds it alters the quaternary structure (due to alterations in the tertiary structure) of the protein which causes haemoglobin to have a higher affinity for the subsequent oxygen molecules and they bind more easily
The existence of the iron II ion (Fe2+) in the prosthetic haem group also allows oxygen to reversibly bind as none of the amino acids that make up the polypeptide chains in haemoglobin are well suited to binding with oxygen
Enzymes
Enzymes are biological catalysts
‘Biological’ because they function in living systems
‘Catalysts’ because they speed up the rate of chemical reactions without being used up or changed
Enzymes are also globular proteins
Critical to the enzyme's function is the active site where the substrate binds
Metabolic pathways are controlled by enzymes in a biochemical cascade of reactions
Virtually every metabolic reaction within living organisms is catalysed by an enzyme – enzymes are therefore essential for life to exist
Enzymes Table

Insulin
The first protein to have its sequence determined by scientists was the hormone insulin
Insulin is a globular protein produced in the pancreas. It plays an important role in the control of blood glucose concentration
It consists of two polypeptide chains
Polypeptide A has 21 amino acid residues
Polypeptide B has 30 amino acid residues
The two polypeptide chains are held together by three disulfide bridges
Examiner Tips and Tricks
Don't forget about how shape relates to function for all of these globular proteins! If their specific shape changes then they can no longer carry out their function.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
