Temperature & Enzyme Activity (Edexcel A Level Biology (A) SNAB) : Revision Note
Temperature & Enzyme Activity
Changing air temperature can have a significant impact on the metabolism of living organisms due to the effect of temperature on enzyme activity
Enzymes have a specific optimum temperature
This is the temperature at which they catalyse a reaction at the maximum rate
Lower temperatures either prevent reactions from proceeding or slow them down
Molecules move relatively slowly as they have less kinetic energy
Less kinetic energy results in a lower frequency of successful collisions between substrate molecules and the active sites of the enzymes which leads to less frequent enzyme-substrate complex formation
Substrates and enzymes also collide with less energy, making it less likely for bonds to be formed or broken
Higher temperatures cause reactions to speed up
Molecules move more quickly as they have more kinetic energy
Increased kinetic energy results in a higher frequency of successful collisions between substrate molecules and the active sites of the enzymes which leads to more frequent enzyme-substrate complex formation
Substrates and enzymes also collide with more energy, making it more likely for bonds to be formed or broken
Denaturation
If temperatures continue to increase past a certain point, the rate at which an enzyme catalyses a reaction drops sharply as the enzymes begin to denature
The increased kinetic energy and vibration of an enzyme puts a strain on its bonds, eventually causing the weaker hydrogen and ionic bonds that hold the enzyme molecule in its precise shape to start to break
The breaking of bonds causes the tertiary structure of the enzyme to change
The active site is permanently damaged and its shape is no longer complementary to the substrate, preventing the substrate from binding
Denaturation has occurred if the substrate can no longer bind

At high temperatures enzymes can denature

The rate of an enzyme catalysed reaction is affected by temperature. Note that 35 C is not the optimum temperature for all enzyme-controlled reactions.
Enzyme activity and living organisms
Changes to enzyme activity that result from changing global temperatures can affect living organisms
Some chemical reactions take place faster at higher temperatures
Photosynthesis is essential for converting carbon dioxide into carbohydrates, the process which produces food for producers and other organisms higher up the food chain; it relies on the function of proteins in the electron transport chain and that of enzymes such as rubisco
E.g. blue-green algae, also known as cyanobacteria, photosynthesise at a higher rate in warmer water due to increased enzyme activity; this increases the formation of potentially harmful algal blooms
Some chemical reactions are slowed down at higher temperatures
At high temperatures plants carry out a reaction called photorespiration at a faster rate; this reaction uses the enzyme rubisco and so slows down photosynthesis
This can reduce crop yields as temperatures rise
Some fish eggs have been shown to develop more slowly at higher temperatures
Many species' successful egg development is dependent on temperature, with impacts such as
Extreme temperature fluctuations can reduce hatching rates in some invertebrates
The sex of the young inside the egg of some species is determined by temperature, so increasing temperatures can affect the sex ratios in a species
E.g. in alligators
Species may have to change their distribution in response to changing temperatures in order to survive
Species may migrate to higher altitudes or further from the equator to find cooler temperatures
Practical: Temperature & Enzyme Activity
The progress of enzyme-catalysed reactions can be investigated by measuring the rate of formation of a product
This can be carried out using the enzyme catalase
Hydrogen peroxide is a common but toxic by-product of cell metabolism which must be broken down quickly
Catalase is an enzyme found in the cells of most organisms that breaks hydrogen peroxide down into water and oxygen
The rate at which oxygen is produced can be recorded to give a measure of enzyme activity
Investigating the effect of temperature on catalase activity
Apparatus
Boiling tubes or flasks
Hydrogen peroxide solution
Buffer solution
Measuring cylinder or syringe
Bung and delivery tube
Water basin
Pipettes
Catalase enzyme solution
Water baths at a range of temperatures
Stopwatch
Method
Set up a series of water baths at different temperatures e.g. 10
C, 20
C, 30
C, 40
C, and 50
C
This provides the varying independent variable
Set up a series of boiling tubes each containing 5 cm3 of hydrogen peroxide solution
Each tube should contain the solution at the same concentration
Equal volumes of a buffer solution can be added to each tube to control pH
Place boiling tubes into water baths at different temperatures and leave for 10 minutes
This allows the tubes to reach the required temperature
Use a pipette to add a sample of catalase solution to a boiling tube containing hydrogen peroxide
Immediately place the bung onto the tube and use the water basin and measuring cylinder to collect the oxygen produced during each 10 second period over the course of 1 minute
The volume of oxygen produced is the dependent variable
Repeat steps 4-5 twice more at the same temperature, ensuring that the same volume of catalase solution is added each time
This allows any anomalous results to be identified
An average volume during each time period can be calculated from the set of results for each temperature
Repeat steps 4-6 using boiling tubes at the remaining temperatures and the same volume of catalase solution
Plot a graph of volume of oxygen produced against time for each temperature tested
Use the graph to calculate the initial rate of reaction for each temperature
Rate of reaction can be calculated using the following equation
rate of reaction = change in volume time taken

The rate of catalase activity can be measured by recording the volume of oxygen produced.
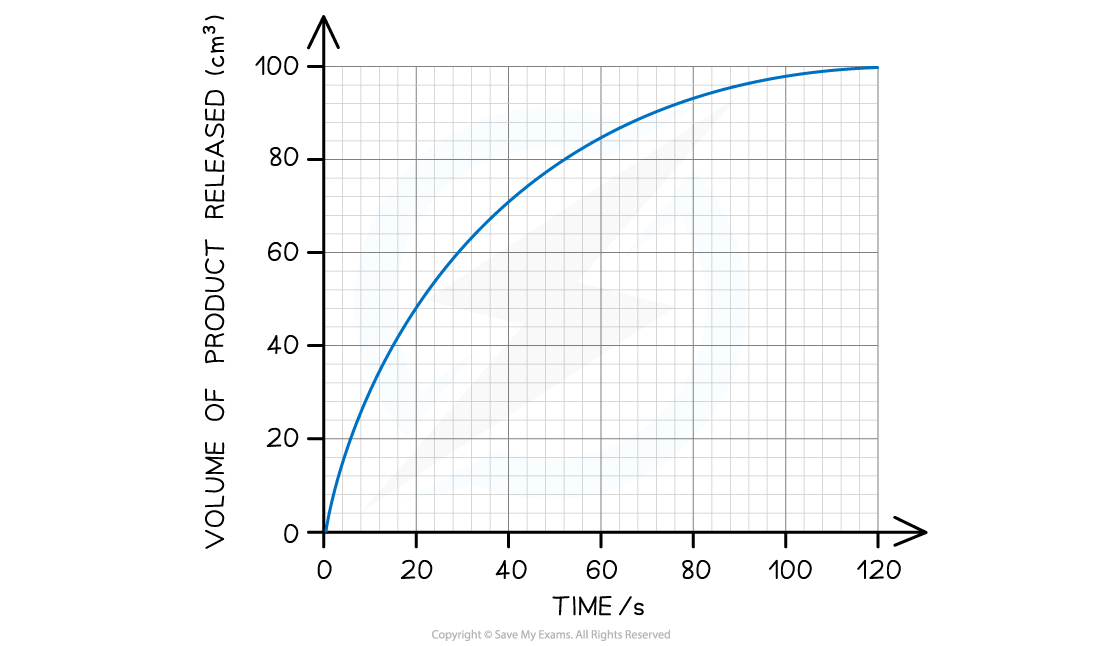
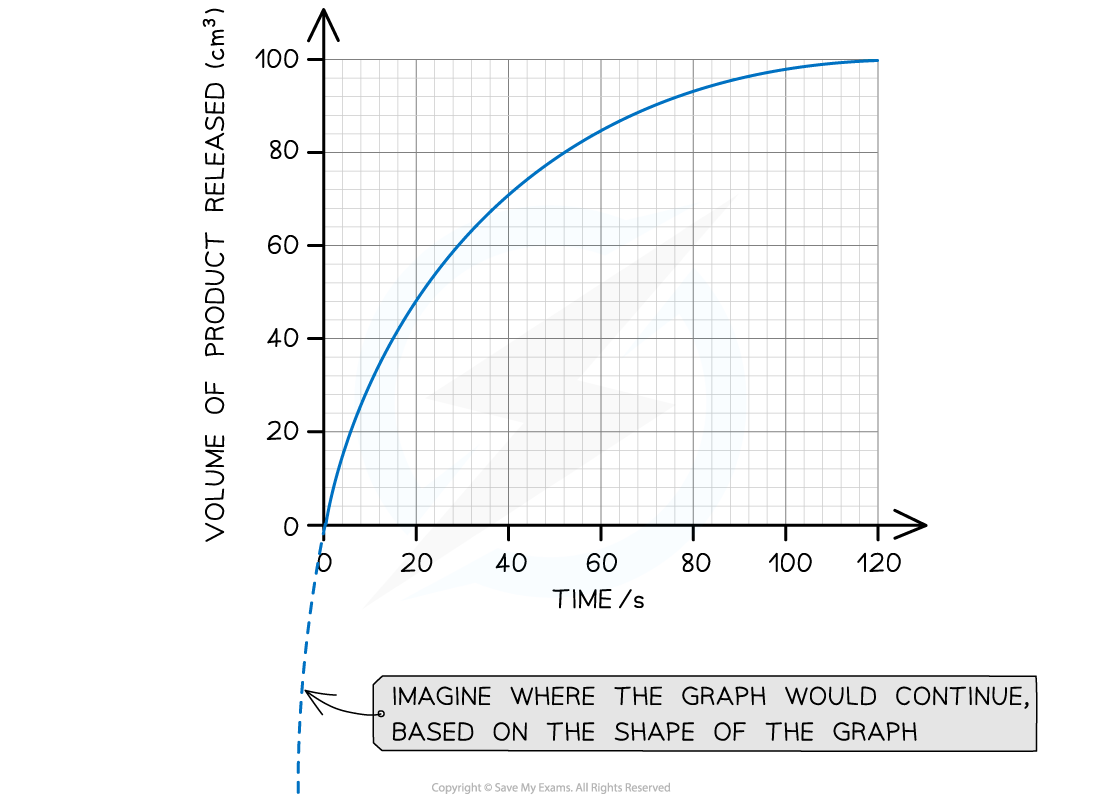
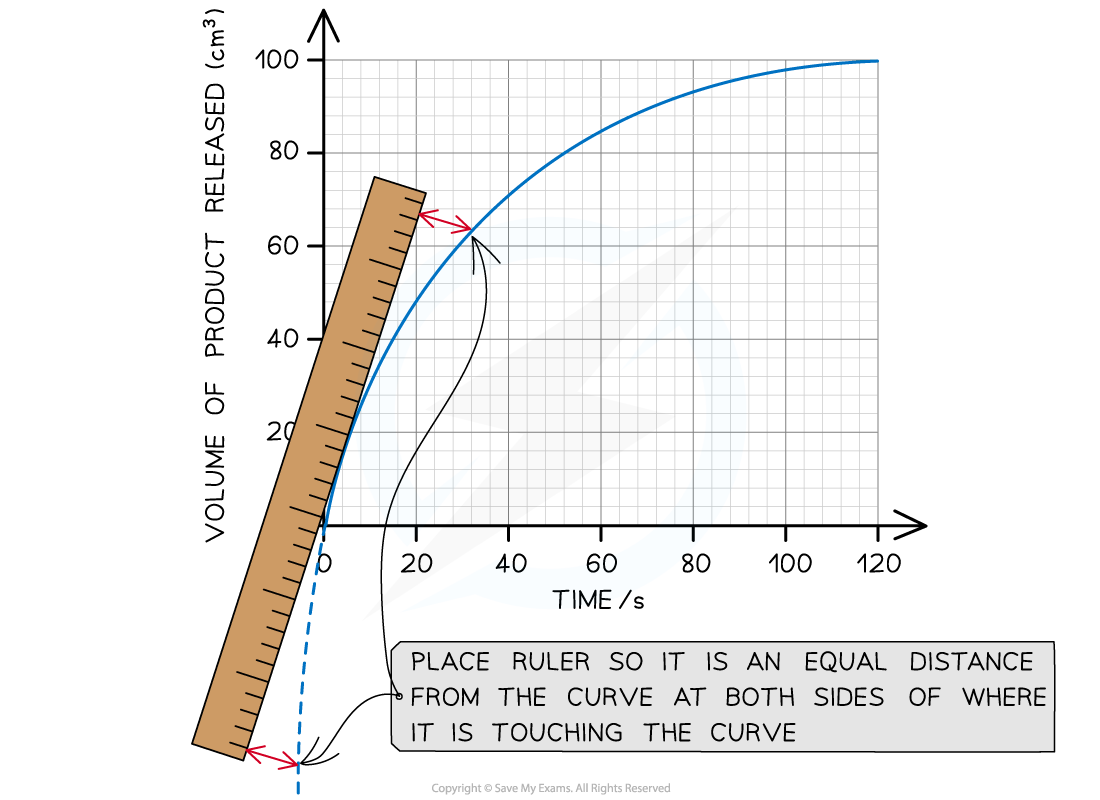
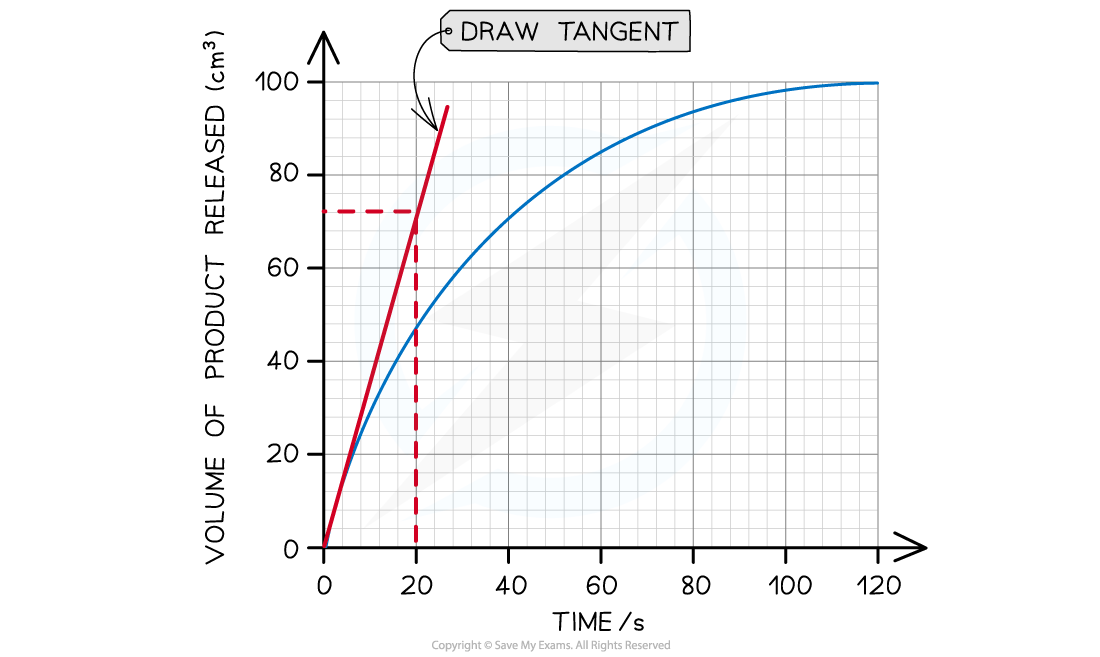

A tangent can be used to calculate the initial rate of an enzyme controlled reaction
Calculating the temperature coefficient
The temperature coefficient, represented by Q10, calculates the increase in rate of reaction when the temperature is increased by 10
C
Q10 can be calculated using the following equation
Q10 = rate at higher temperature rate at lower temperature
A Q10 value of 2 indicates that the reaction rate doubles with an increase in temperature of 10
C, while a value of 3 indicates that it trebles with every 10
C increase
Worked Example
In an enzyme catalysed reaction the rate of reaction can measured by recording the volume of product produced per unit time at different temperatures.
At 30 C 3.5 cm3 s-1 of product was recorded and at 40
C 6.8 cm3 s-1 was recorded. Calculate Q10 for this reaction.
Answer:
Step 1: Write out the relevant equation
Q10 = rate at higher temperature rate at lower temperature
Step 2: Substitute numbers into the equation
Q10 = 6.8 3.5
Step 3: Complete calculation
Q10 = 1.94
This value is close to 2, indicating that the rate of reaction has almost doubled

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
