Fibrous Proteins: Structure & Function (Edexcel A Level Biology (A) SNAB) : Revision Note
Fibrous Proteins: Structure & Function
Structure
Fibrous proteins are long strands of polypeptide chains that have cross-linkages due to hydrogen bonds
These proteins have little or no tertiary structure
Fibrous proteins have a limited number of amino acids with the sequence usually being highly repetitive
The highly repetitive sequence creates very organised structures
Function
Due to a large number of hydrophobic R groups, fibrous proteins are insoluble in water
Fibrous proteins are strong and this, along with their insolubility property, makes fibrous proteins very suitable for structural roles
Examples of fibrous proteins:
Keratin makes up hair, nails, horns and feathers (it is a very tough fibrous protein)
Elastin is found in connective tissue, tendons, skin and bone (it can stretch and then return to its original shape)
Collagen is a connective tissue found in skin, tendons and ligaments
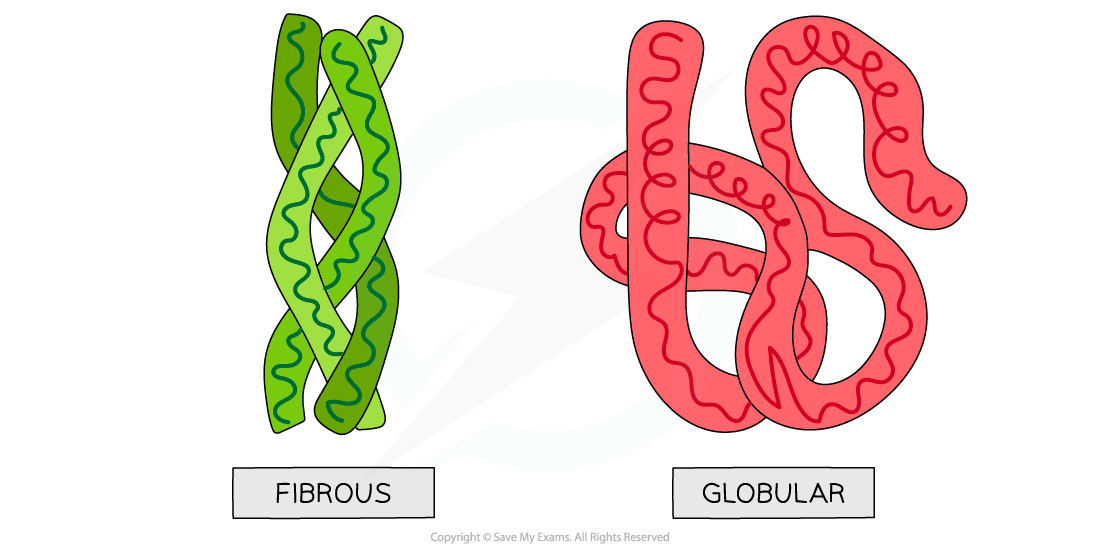
Globular and fibrous protein models illustrating the spherical shape of globular proteins and the long, stranded shape of fibrous proteins.
Collagen
Collagen is the most common structural protein found in vertebrates
It provides structural support
In vertebrates it is the component of connective tissue which forms:
Tendons
Cartilage
Ligaments
Bones
Teeth
Skin
Walls of blood vessels
Cornea of the eye
Collagen is an insoluble fibrous protein
Structure of collagen
Collagen is formed from three polypeptide chains closely held together by hydrogen bonds to form a triple helix (known as tropocollagen)
Each polypeptide chain is a helix shape (but not α-helix as the chain is not as tightly wound) and contains about 1000 amino acids with glycine, proline and hydroxyproline being the most common
In the primary structure of collagen almost every third amino acid is glycine
This is the smallest amino acid with a R group that contains a single hydrogen atom
Glycine tends to be found on the inside of the polypeptide chains allowing the three chains to be arranged closely together forming a tight triple helix structure
Along with hydrogen bonds forming between the three chains there are also covalent bonds present
Covalent bonds also form cross-links between R groups of amino acids in interacting triple helices when they are arranged parallel to each other
The cross-links hold the collagen molecules together to form fibrils
The collagen molecules are positioned in the fibrils so that there are staggered ends (this gives the striated effect seen in electron micrographs)
When many fibrils are arranged together they form collagen fibres
Collagen fibres are positioned so that they are lined up with the forces they are withstanding
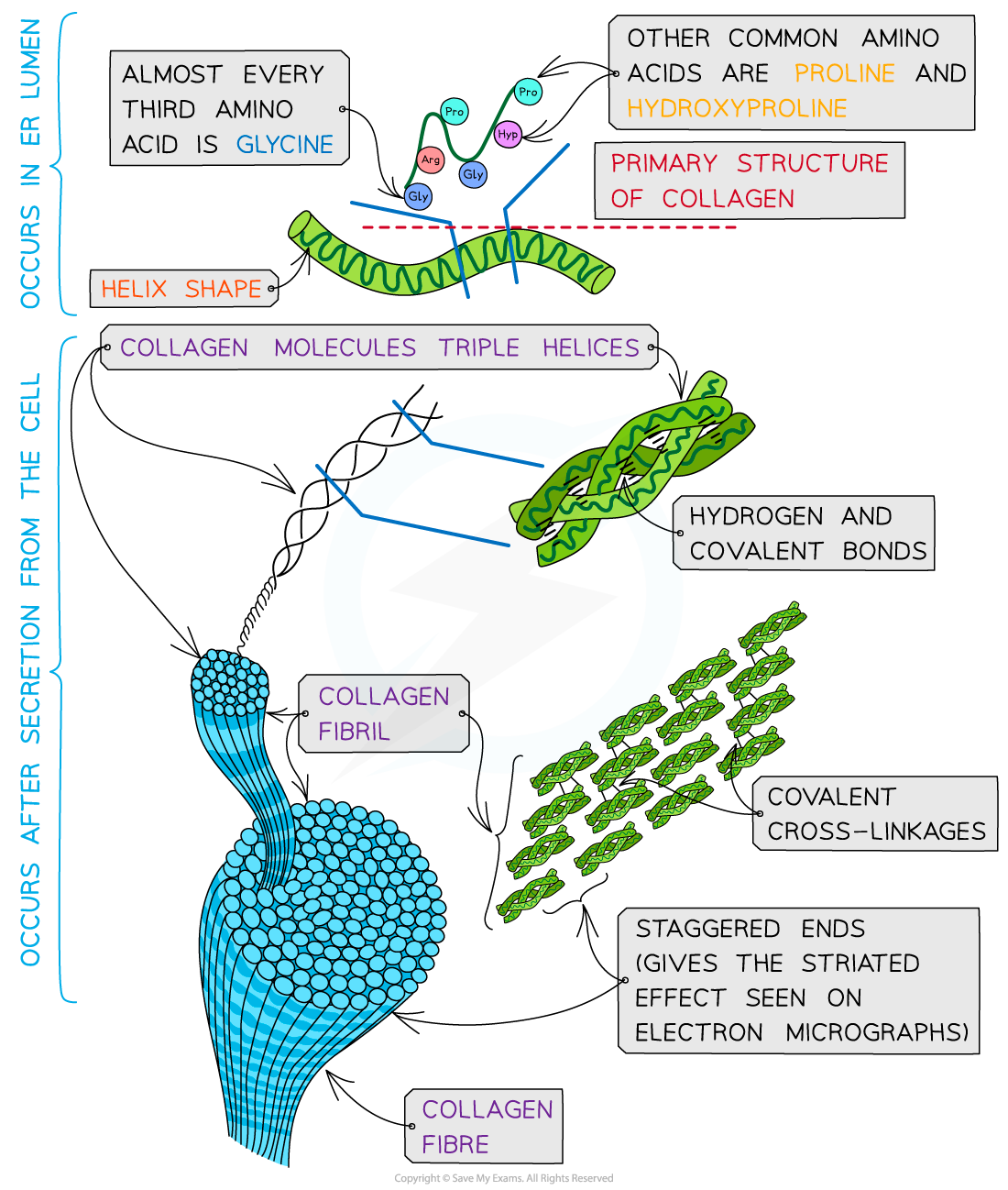
Collagen is a fibrous structural protein that is formed by triple helices. Collagen molecules arrange into collagen fibrils and finally into collagen fibres which have high tensile strength
Function of collagen
Collagen is a flexible structural protein forming connective tissues
The presence of the many hydrogen bonds within the triple helix structure of collagen results in great tensile strength. This enables collagen to be able to withstand large pulling forces without stretching or breaking
The staggered ends of the collagen molecules within the fibrils provide strength
Collagen is a stable protein due to the high proportion of proline and hydroxyproline amino acids present. These amino acids increase stability as their R groups repel each other
The length of collagen molecules means they take too long to dissolve in water (making it insoluble in water)
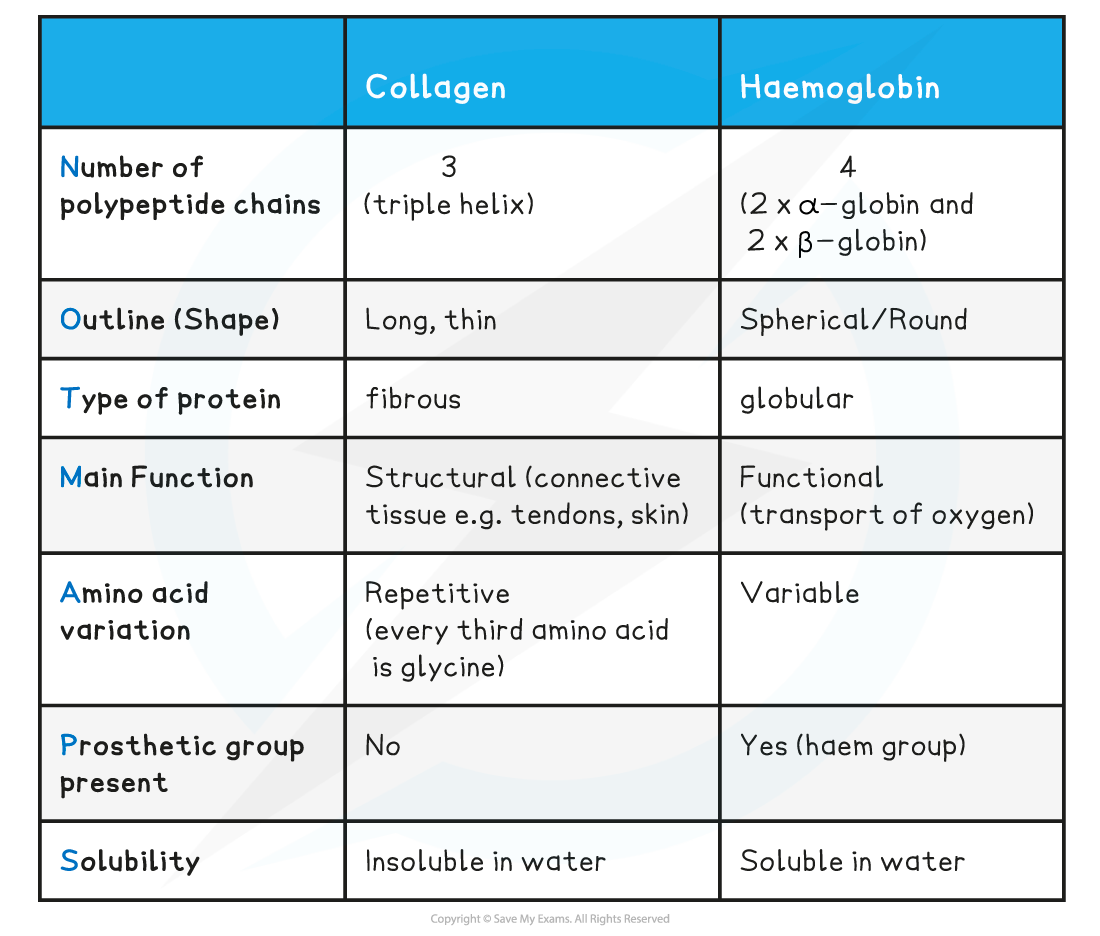
Comparison of Collagen and Haemoglobin Table

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
