The Role of Water in Living Organisms (Cambridge (CIE) A Level Biology): Revision Note
Exam code: 9700
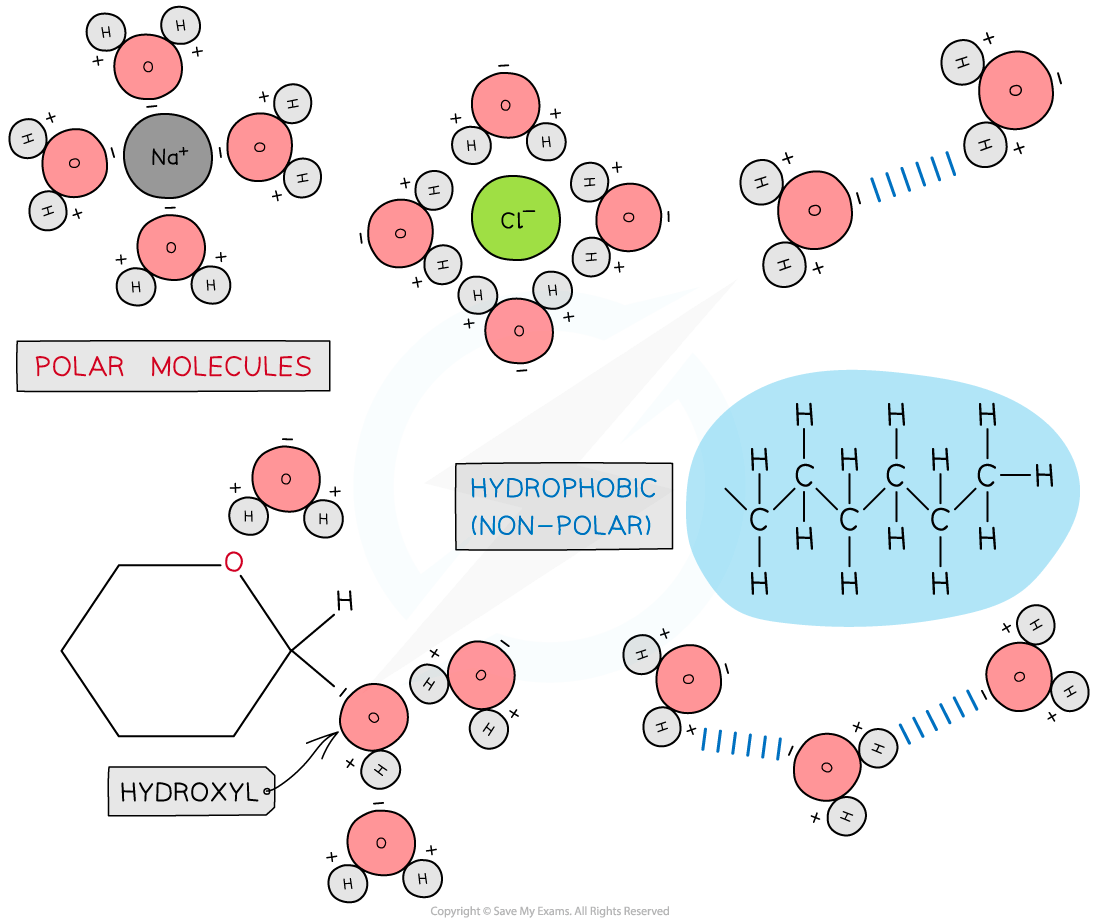
Water molecules: in living organisms
Water has many essential roles in living organisms due to its properties:
The polarity of water molecules
The presence and number of hydrogen bonds between water molecules
Solvent
As water is a polar molecule many ionic compounds (e.g. sodium chloride) and covalently bonded polar substances (e.g. glucose) will dissolve in it
This allows chemical reactions to occur within cells (as the dissolved solutes are more chemically reactive when they are free to move about)
Metabolites can be transported efficiently (except non-polar molecules which are hydrophobic)
High specific heat capacity
The specific heat capacity of a substance is the amount of thermal energy required to raise the temperature of 1kg of that substance by 1°C. Water’s specific heat capacity is 4200 J/kg°C
The high specific heat capacity is due to the many hydrogen bonds present in water
It takes a lot of thermal energy to break these bonds and a lot of energy to create them, thus the temperature of water does not fluctuate greatly
The advantage for living organisms is that it:
Provides suitable habitats
Allows for constant temperatures within bodies and cells to be maintained (this ensures enzymes have the optimal temperatures)
This is because a large increase in energy is needed to increase the temperature of water
Latent heat of vaporisation
In order to change state (from liquid to gas) a large amount of thermal energy must be absorbed by water to break the hydrogen bonds and evaporate
This is an advantage for living organisms as only a little water is required to evaporate from the surface of the organism in order to lose a great amount of heat energy
This provides a cooling effect for living organisms, for example the transpiration from leaves or evaporation of water in sweat from the skin
Property | Role in Living Organisms | Reason |
|---|---|---|
Solvent | Allows chemical reactions to occur Transport medium | Polarity of water |
High specific heat capacity | Allows water to be a suitable habitat Optimal temperature maintained within cells and bodies | Presence of many hydrogen bonds |
High latent heat of vaporisation | Coolant | Presence of many hydrogen bonds |
Examiner Tips and Tricks
When discussing the role water has in living organisms remember to mention the ‘why’ in relation to its properties (i.e. it is an excellent solvent because of to the polar nature of water molecules).

Unlock more, it's free!
Did this page help you?