Amino Acids & the Peptide Bond (Cambridge (CIE) A Level Biology) : Revision Note
Amino Acids & the Peptide Bond
Proteins
Proteins are polymers (and macromolecules) made of monomers called amino acids
The sequence, type and number of the amino acids within a protein determines its shape and therefore its function
Proteins are extremely important in cells because they form all of the following:
Enzymes
Cell membrane proteins (e.g. carrier)
Hormones
Immunoproteins (e.g immunoglobulins)
Transport proteins (e.g haemoglobin)
Structural proteins (e.g keratin, collagen)
Contractile proteins (e.g. myosin)
Amino acids
Amino acids are the monomers of proteins
There are 20 amino acids found in proteins common to all living organisms
The general structure of all amino acids is a central carbon atom bonded to:
An amine group -NH2
A carboxylic acid group -COOH
A hydrogen atom
An R group (which is how each amino acid differs and why amino acid properties differ e.g. whether they are acidic or basic or whether they are polar or non-polar)
Amino Acid Structure Diagram

The generalised structure of an amino acid
The peptide bond
In order to form a peptide bond a hydroxyl group (-OH) is lost from the carboxylic group of one amino acid and a hydrogen atom is lost from the amine group of another amino acid
The remaining carbon atom (with the double-bonded oxygen) from the first amino acid bonds to the nitrogen atom of the second amino acid
This is a condensation reaction so water is released
The resulting molecule is a dipeptide
When many amino acids are bonded together by peptide bonds the molecule formed is called a polypeptide. A protein may have only one polypeptide chain or it may have multiple chains interacting with each other
During hydrolysis reactions polypeptides are broken down to amino acids when the addition of water breaks the peptide bonds
Peptide Bond Formation
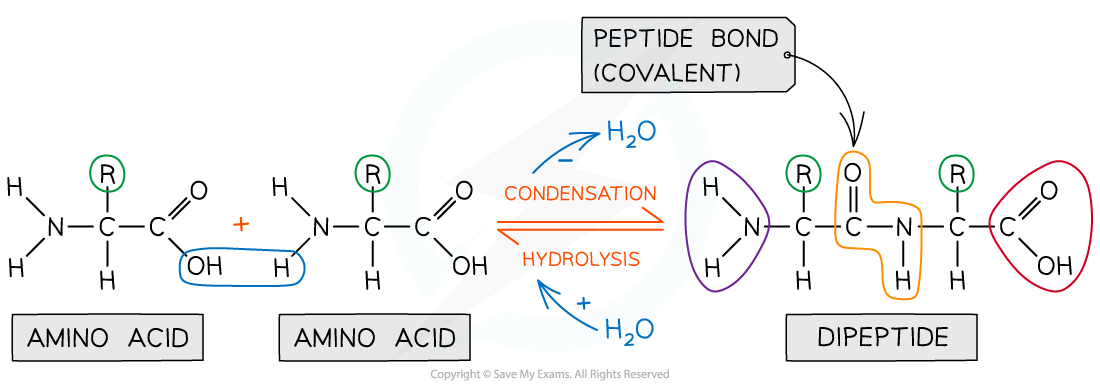
Amino acids are bonded together by covalent peptide bonds to form a dipeptide in a condensation reaction
Examiner Tips and Tricks
You will be expected to recognise whether an unfamiliar molecule is an amino acid or protein so look for the functional groups (amine and carboxyl).
When asked to identify the location of the peptide bond, look for where nitrogen is bonded to a carbon which has a double bond with an oxygen atom, note the R group is not involved in the formation of a peptide bond.
You will also be expected to draw out the general structure of an amino acid so be sure to practise this skill lots.

You've read 0 of your 5 free revision notes this week
Sign up now. It’s free!
Did this page help you?
