Investigating the Rate of Photosynthesis (Cambridge (CIE) A Level Biology): Revision Note
Exam code: 9700
Investigating the rate of photosynthesis: redox indicators
The light-dependent reactions of photosynthesis take place in the thylakoid membrane and involve the release of high-energy electrons from chlorophyll a molecules
These electrons are picked up by electron acceptors and then passed down the electron transport chain
However, if a redox indicator (such as DCPIP or methylene blue) is present, the indicator takes up the electrons instead
This causes the indicator to change colour
DCPIP: oxidised (blue) → accepts electrons → reduced (colourless)
Methylene blue: oxidised (blue) → accepts electrons → reduced (colourless)
The colour of the reduced solution may appear green because the chlorophyll has a green colour
The rate at which the redox indicator changes colour from its oxidised state to its reduced state can be used as a measure of the rate of photosynthesis
When light is at a higher intensity, or at more preferable light wavelengths, the rate of photoactivation of electrons is faster, therefore the rate of reduction of the indicator is faster
Method
Leaves are crushed in a liquid known as an isolation medium
This produces a concentrated leaf extract that contains a suspension of intact and functional chloroplasts
The medium must have the same water potential as the leaf cells (so the chloroplasts don’t shrivel or burst) and contain a buffer (to keep the pH constant)
It should also be ice cold (to avoid damaging the chloroplasts and to maintain membrane structure)
Small tubes are set up with different intensities, or different colours (wavelengths) of light shining on them
If different intensities of light are used, they must all be of the same wavelength (same colour of light)
If different wavelengths of light are used, they must all be of the same light intensity
DCPIP of methylene blue indicator is added to each tube, as well as a small volume of the leaf extract
The time taken for the redox indicator to go colourless is recorded
This is a measure of the rate of photosynthesis
Examiner Tips and Tricks
In chemistry, the acronym ‘OIL RIG’ is used to remember if something is being oxidised or reduced.
Oxidation Is Loss (of electrons)
Reduction Is Gain (of electrons)
Therefore DCPIP in its oxidised state has not accepted electrons and in its reduced state has accepted electrons.
Investigating the rate of photosynthesis: aquatic plants
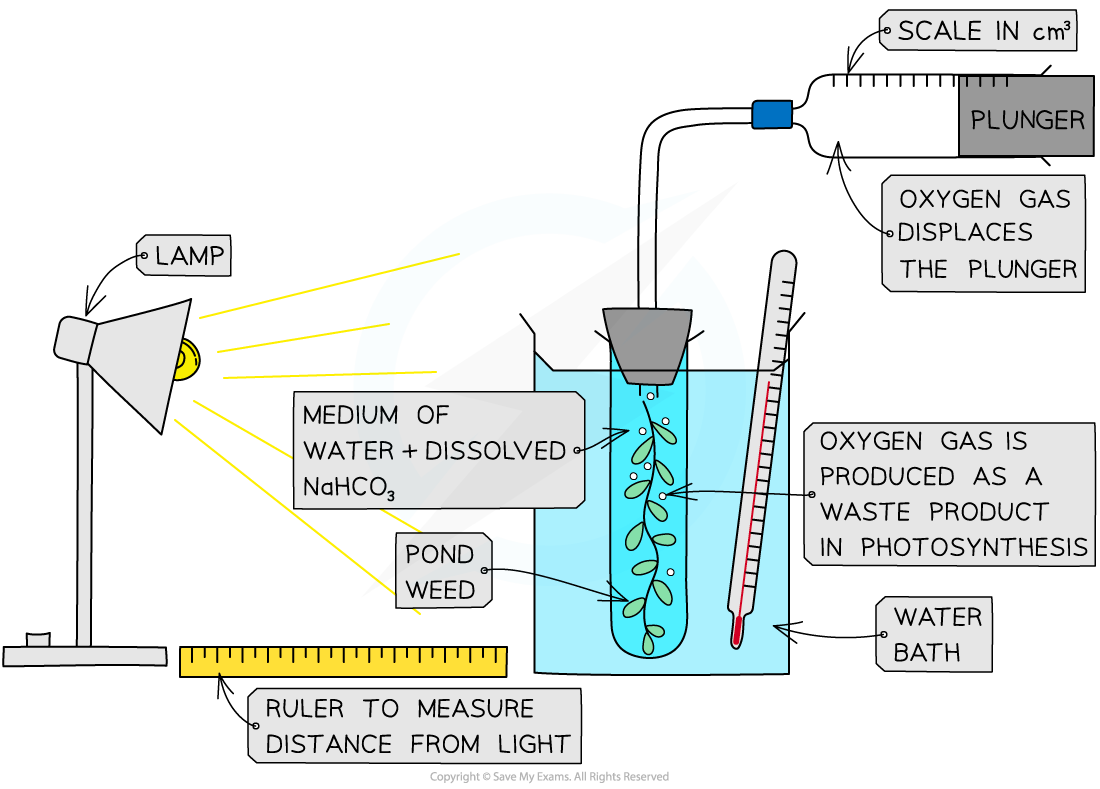
Investigations to determine the effects of light intensity, carbon dioxide concentration and temperature on the rate of photosynthesis can be carried out using aquatic plants, such as Elodea or Cabomba (types of pondweed)
The effect of these limiting factors on the rate of photosynthesis can be investigated in the following ways:
Light intensity—change the distance (d) of a light source from the plant (light intensity is proportional to 1/d2)
Carbon dioxide concentration—add different quantities of sodium hydrogencarbonate (NaHCO3) to the water surrounding the plant, this dissolves to produce CO2
Temperature (of the solution surrounding the plant)—place the boiling tube containing the submerged plant in water baths of different temperatures
Whilst changing one of these factors during the investigation (as described below), ensure the other two remain constant
For example, when investigating the effect of light intensity on the rate of photosynthesis, a glass tank should be placed in between the lamp and the boiling tube containing the pondweed to absorb heat from the lamp – this prevents the solution surrounding the plant from changing temperature
Method
Ensure that the water is well aerated before use by bubbling air through it
This will ensure that oxygen gas given off by the plant during the investigation forms bubbles and does not dissolve in the water
Ensure that the plant has been well illuminated before starting the experiment
This will ensure that the plant contains all the enzymes required for photosynthesis and that any changes in rate are due to the independent variable rather than an increase in enzyme activity
Cut the stem of the pondweed cleanly just before placing it into the boiling tube
Cutting the stem at an angle provides a larger surface area from which bubbles can form
Set up the apparatus (as shown below) in a darkened room
This ensures that the lamp is the only light source and so allows light intensity to be controlled
Ensure that the pondweed is fully submerged in sodium hydrogencarbonate solution (1%); this ensures that the pondweed has a controlled supply of carbon dioxide for photosynthesis
Measure the volume of gas collected in the gas syringe in a set period of time, e.g. 5 minutes
Repeat step 5 at least twice more
Change the independent variable and repeat step 5 again
The independent variable could be the light intensity, carbon dioxide concentration or temperature depending on which limiting factor you are investigating
Record the results in a table and plot a graph of the volume of oxygen produced per minute against the independent variable
Examiner Tips and Tricks
Learn the 3 limiting factors and how each one can be altered in a laboratory environment:
Light intensity – the distance of the light source from the plant (intensity ∝ 1/d2)
Temperature - changing the temperature of the water bath the test tube sits in
Carbon dioxide - the concentration of NaHCO3 dissolved in the water the pondweed is in
Also, remember that the variables not being tested (the control variables) must be kept constant.

Unlock more, it's free!
Did this page help you?