Respiratory Quotient (RQ) (Cambridge (CIE) A Level Biology): Revision Note
Exam code: 9700
Respiratory quotient (RQ)
The respiratory quotient (RQ) is: the ratio of carbon dioxide molecules produced to oxygen molecules taken in during respiration
RQ values of different respiratory substrates
Carbohydrates, lipids and proteins have different typical RQ values
This is because the number of carbon-hydrogen bonds differs in each type of biological molecule
A higher number of carbon-hydrogen bonds means that more hydrogen atoms can be used to create a proton gradient
More hydrogens means that more ATP molecules can be produced by chemiosmosis
More oxygen is therefore required to break down the molecule (in the last step of oxidative phosphorylation to form water)
When glucose is respired aerobically, equal volumes of carbon dioxide are produced and oxygen taken in, meaning it has an RQ value of 1

Respiratory substrate | Typical RQ value |
|---|---|
Carbohydrate | 1.0 |
Protein | 0.8 - 0.9 |
Lipid | 0.7 |
Examiner Tips and Tricks
Some questions may ask you to suggest what substrate is being respired during an experiment based on the RQ value—so make yourself familiar with the values in the table.
Calculating RQs
The respiratory quotient is calculated from respiration equations
It involves comparing the ratios of carbon dioxide given out to oxygen taken in
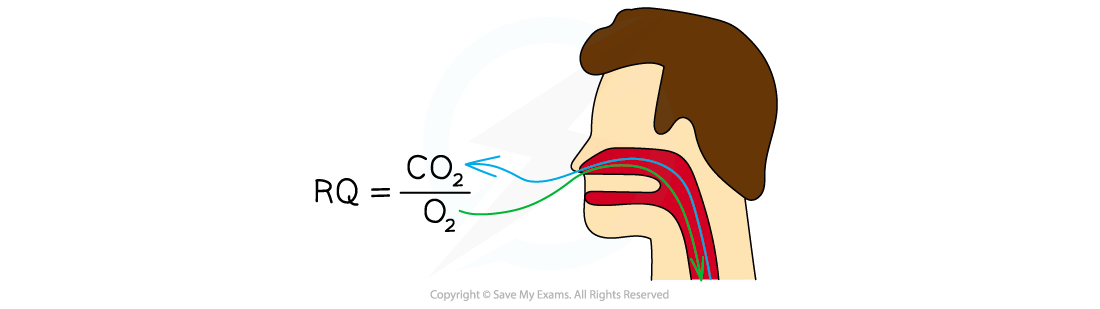
The formula for this is:

Equation to calculate the RQ
If you know the molecular formula of the substrate being aerobically respired then you can create a balanced equation to calculate the RQ value
In a balanced equation the number before the chemical formula can be taken as the number of molecules/moles of that compound
This is because the same number of molecules of any gas take up the same volume e.g. 12 molecules of carbon dioxide take up the same volume as 12 molecules of oxygen
Glucose has a simple 1:1 ratio and RQ value of 1 but other substrates have more complex ratios leading to different RQ values
Worked Example
RQ for a lipid
Linoleic acid (a fatty acid found in nuts) has the molecular formula C18H32O2
Step 1: Create a respiration equation
C18H32O2 + O2 → CO2 + H2O
Step 2: Balance the equation
C × 18 C × 1
H × 32 H × 2
O × 4 O × 3
Step 3: Create the full equation
C18H32O2 + 25O2 → 18CO2 + 16H2O
Step 3: Use the RQ formula
Calculating the RQ for anaerobic respiration
Anaerobic respiration is respiration that takes place without oxygen but does produce a small amount of ATP
Depending on the organism anaerobic respiration in cells can be done via lactate or ethanol fermentation
Mammalian muscle cells use lactate fermentation
Plant tissue cells and yeast use ethanol fermentation
The RQ cannot be calculated for anaerobic respiration in muscle cells because no oxygen is used and no carbon dioxide is produced during lactate fermentation
For yeast cells the RQ tends towards infinity as no oxygen is used while carbon dioxide is still being produced
Worked Example
RQ for Anaerobic Respiration
Ethanol fermentation in lettuce roots
glucose → ethanol + carbon dioxide (+ energy)
Step 1: Create the respiration equation
C6H12O6 → C2H5OH + CO2 (+ energy)
Step 2: Balance the equation
C6H12O6 → 2C2H5OH + 2CO2 + energy
Step 3: Use the RQ formula
Examiner Tips and Tricks
Make sure the respiration equation you are working with is fully balanced before you start doing any calculations to find out the RQ value.

Unlock more, it's free!
Did this page help you?