Chromatography of Chloroplast Pigments (Cambridge (CIE) A Level Biology): Revision Note
Exam code: 9700
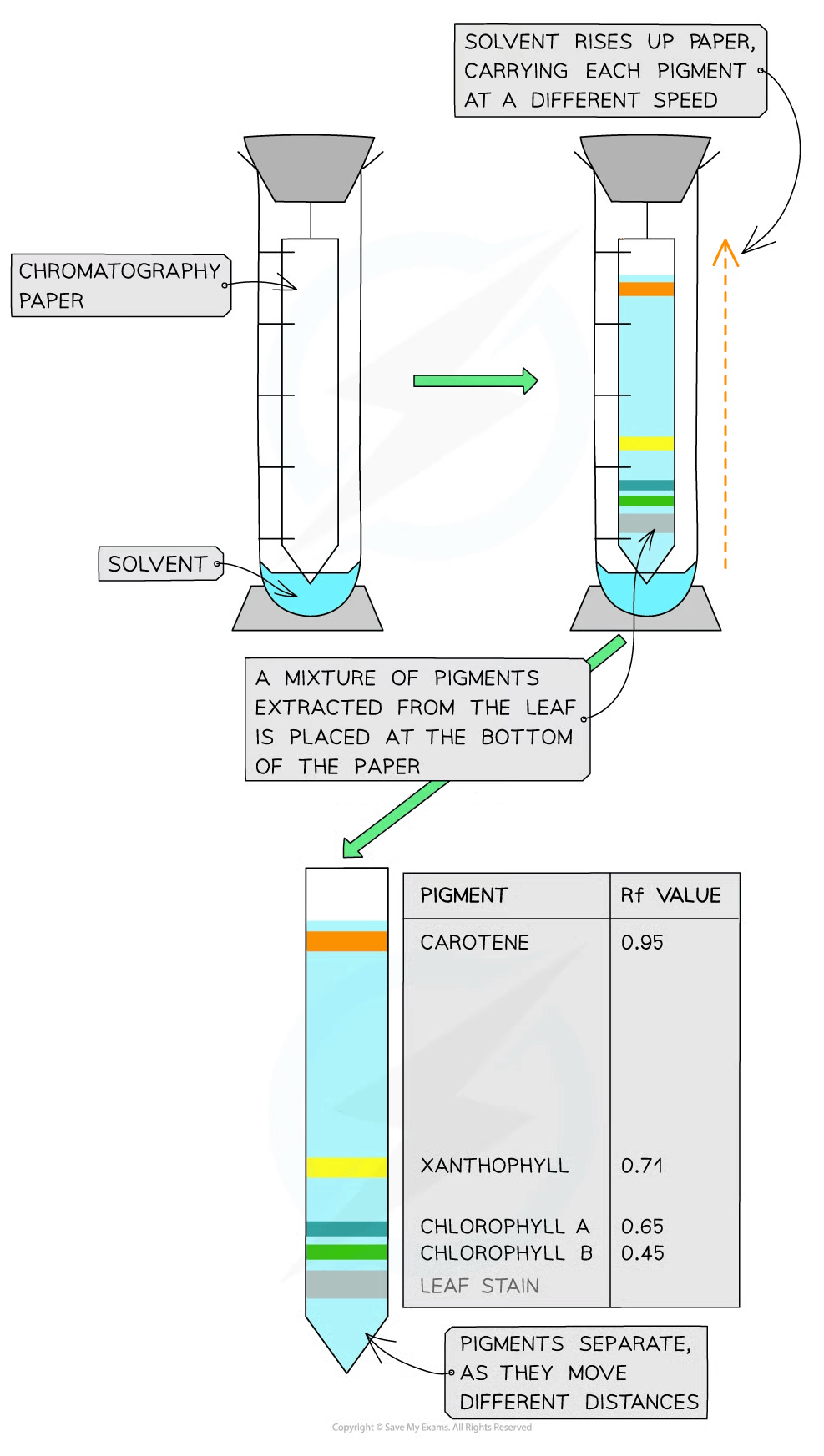
Chromatography of chloroplast pigments
Chromatography is an experimental technique that is used to separate mixtures:
The mixture is dissolved in a fluid/solvent (called the mobile phase) and the dissolved mixture then passes through a static material (called the stationary phase)
Different components within the mixture travel through the material at different speeds
This causes the different components to separate
A retardation factor (Rf) can be calculated for each component of the mixture
Rf value = distance travelled by component ÷ distance travelled by solvent
Two of the most common techniques for separating these photosynthetic pigments are:
Paper chromatography—the mixture of pigments is passed through paper (cellulose)
Thin layer chromatography—the mixture of pigments is passed through a thin layer of adsorbent (e.g. silica gel), through which the mixture travels faster and separates more distinctly
Chromatography can be used to separate and identify chloroplast pigments that have been extracted from a leaf, as each pigment will have a unique Rf value
The Rf value demonstrates how far a dissolved pigment travels through the stationary phase
A smaller Rf value indicates the pigment is less soluble and larger
Although specific Rf values depend on the solvent that is being used, in general:
Carotenoids have the highest Rf values (usually close to 1)
Chlorophyll B has a much lower Rf value
Chlorophyll A has an Rf value somewhere between those of carotenoids and chlorophyll B
Small Rf values indicate the pigment is less soluble and larger
Examiner Tips and Tricks
Make sure you learn the approximate Rf values for the different pigments within chloroplasts (or at least their values relative to each other). This means you should be able to identify different chloroplast pigments based on their Rf values alone.

Unlock more, it's free!
Was this revision note helpful?
