ATP (Cambridge (CIE) A Level Biology): Revision Note
Exam code: 9700
ATP: universal energy currency
The energy released during the reactions of respiration is transferred to the molecule adenosine triphosphate (ATP)
ATP is a small, soluble molecule that provides a short-term store of chemical energy that cells can use to do work
It is vital in linking energy-requiring and energy-yielding reactions
ATP is described as a universal energy currency
Universal: it is used in all organisms
Currency: like money, it can be used for different purposes (reactions) and is reused countless times
The use of ATP as an ‘energy-currency’ is beneficial for many reasons:
The hydrolysis of ATP can be carried out quickly and easily wherever energy is required within the cell by the action of just one enzyme, ATPase
A useful (not too small, not too large) quantity of energy is released from the hydrolysis of one ATP molecule—this is beneficial as it reduces waste but also gives the cell control over what processes occur
ATP is relatively stable at cellular pH levels
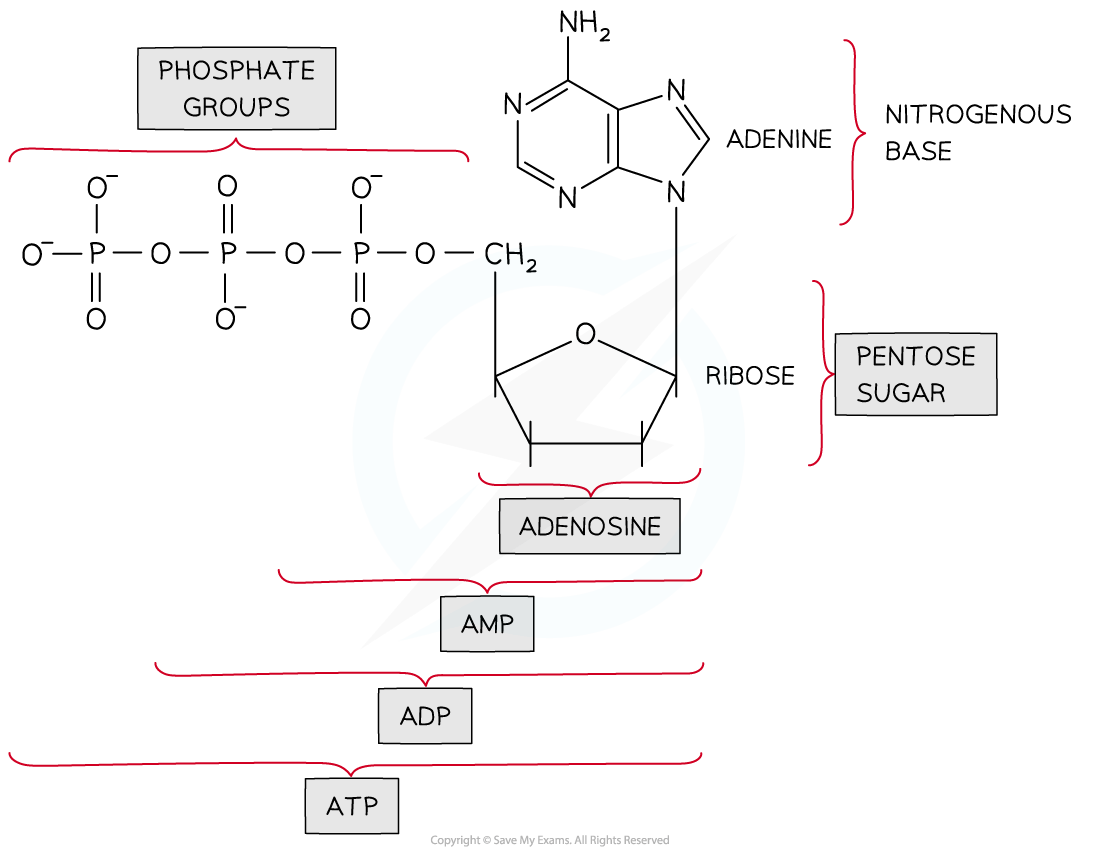
Structure of ATP
ATP is a phosphorylated nucleotide
It is made up of:
Ribose sugar
Adenine base
Three phosphate groups
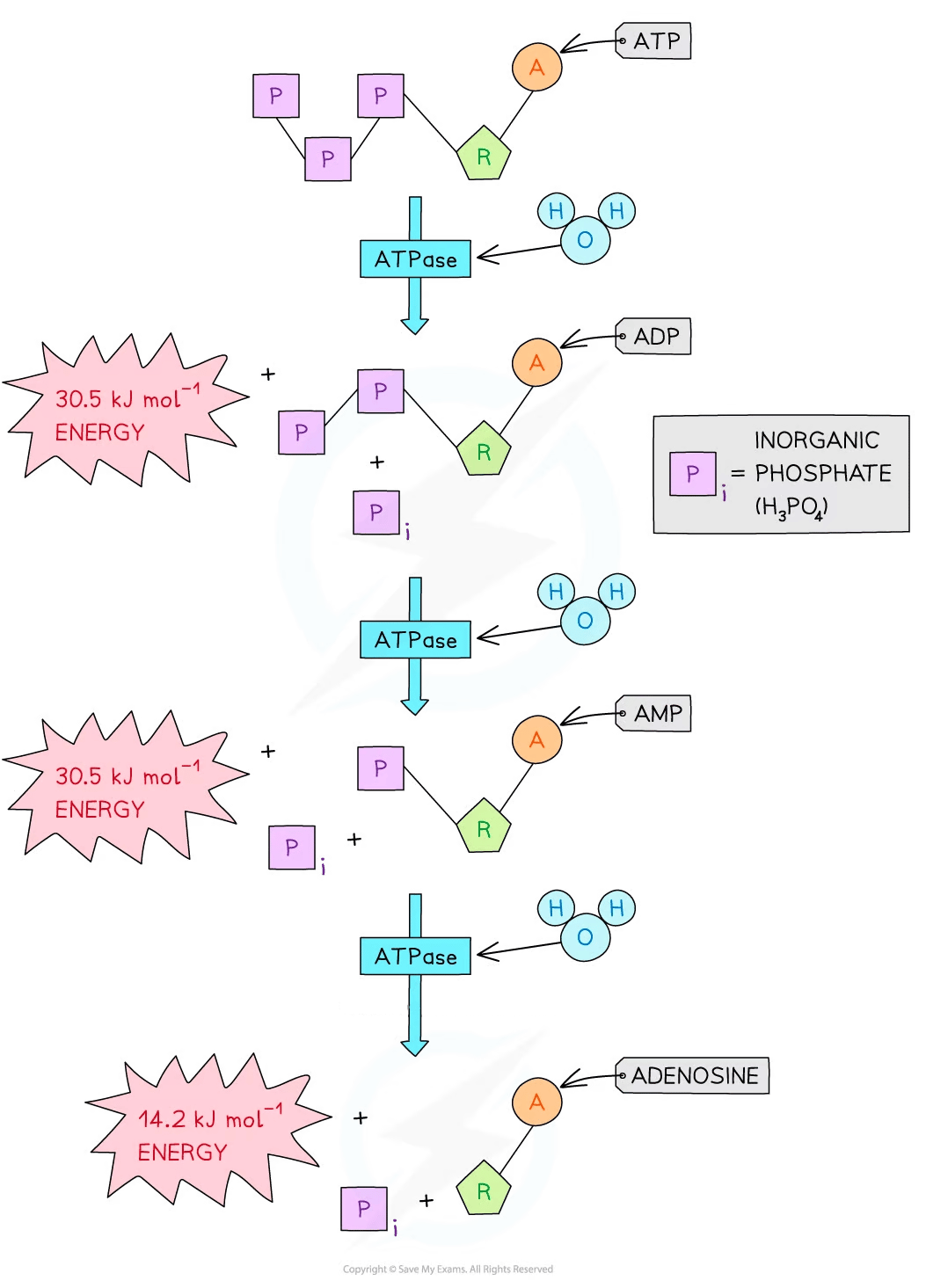
Hydrolysis of ATP
When ATP is hydrolysed (broken down), ADP and phosphate are produced
As ADP is formed, free energy is released that can be used for processes within a cell e.g. DNA synthesis
Removal of one phosphate group from ATP releases approximately 30.5 kJ mol -1 of energy, forming ADP
Removal of a second phosphate group from ADP also releases approximately 30.5 kJ mol-1 of energy, forming AMP
Removal of the third and final phosphate group from AMP releases 14.2 kJ mol-1 of energy, forming adenosine
Feature | Benefit |
|---|---|
Releases a small but sufficient amount of energy (75.2 kJ mol-1 from the complete hydrolysis of ATP) | This is enough energy to drive important metabolic reactions while keeping energy wastage low |
Exists as a stable molecule | It does not break down unless a catalyst (ATPase) is present, so energy will not be wasted |
Can be recycled | The breakdown of ATP is a reversible reaction; ATP can be reformed from ADP and Pi. This means that the same molecule can be used elsewhere in the cell for different reactions |
Hydrolysis is quick and easy | Allows cells to respond to a sudden increase in energy demand |
Soluble and moves easily within a cell | Can transport energy to different areas of the cell |
Forms phosphorylated intermediates | This can make metabolites more reactive and lower the activation energy required for a reaction |
Examiner Tips and Tricks
Be careful not to use the terms energy and ATP interchangeably. Energy is the capacity or power to do work. ATP is a molecule which stores (chemical potential) energy and carries it to places in the cell that need energy to do work. For example, it is correct to say that respiration 'produces ATP', but you should never say that it 'produces energy'.
ATP synthesis
Organisms cannot build up large stores of ATP
This means the cells must make ATP as and when they need it
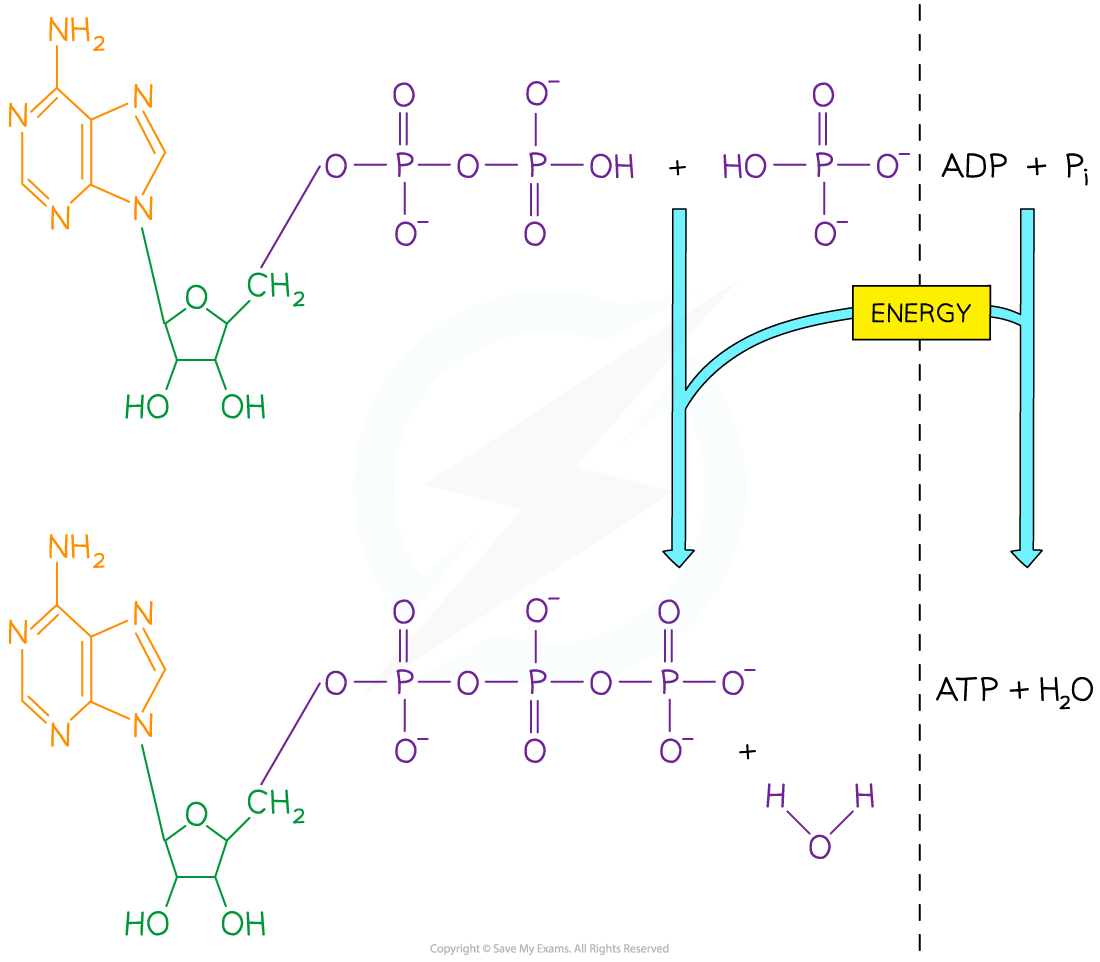
ATP is formed when ADP is combined with an inorganic phosphate (Pi) group
This is an energy-requiring reaction
Water is released as a waste product (therefore ATP synthesis is a condensation reaction)
Types of ATP synthesis
ATP is made during the reactions of respiration and photosynthesis
All of an animal's ATP comes from respiration
ATP can be made in two different ways:
Substrate-linked phosphorylation
Chemiosmosis
Substrate-linked phosphorylation
ATP is formed by transferring a phosphate directly from a substrate molecule to ADP
ADP + Pi → ATP
The energy required for the reaction is provided directly by another chemical reaction
This type of ATP synthesis occurs in the cell cytoplasm and in the matrix of the mitochondria
It only accounts for a small amount of the ATP synthesised during aerobic respiration
~ 4 / 6 ATP per glucose molecule
This type of ATP synthesis takes place in glycolysis
Chemiosmosis
This specific type of ATP synthesis involves a proton (hydrogen ion) gradient across a membrane
It takes place across the inner membrane of the mitochondria and the thylakoid membrane of chloroplasts
An electron transport chain helps to establish the proton concentration gradient
High-energy electrons move from carrier to carrier releasing energy that is used to pump protons (up a concentration gradient) across the inner membrane into the intermembrane space
Protons are pumped from a low concentration in the mitochondrial matrix to a high concentration in the intermembrane space
The protons then move down the concentration gradient into the matrix which releases energy
The protons move through the ATP synthase complex which uses the released energy to drive the phosphorylation of ATP
Oxygen acts as the final electron and proton acceptor to form water
Most of the ATP made during respiration is synthesised via chemiosmosis
~ 32 / 34 ATP per glucose molecule
| Substrate-linked phosphorylation | Chemiosmosis |
|---|---|---|
Process | The phosphate of a substrate molecule is transferred directly to ADP to form ATP. It uses the energy provided directly by another chemical reaction. | The energy released by the movement of hydrogen ions down a concentration gradient is used to synthesise ATP via the enzyme ATP synthase. Oxygen acts as the final hydrogen/electron acceptor |
Location | The cytoplasm of cells / the matrix of mitochondria | Inner mitochondrial membrane / thylakoid membrane of chloroplasts |
Quantity of ATP produced during respiration | Small (4/6 per glucose molecule respired) | Large (32/34 per glucose molecule respired) |
Examiner Tips and Tricks
You may be asked to identify which type of ATP synthesis is occurring at different stages of respiration and photosynthesis. Remember that chemiosmosis involves a proton gradient that has been created by an electron transport chain and it takes place across an inner membrane.

Unlock more, it's free!
Did this page help you?