Haemoglobin & Oxygen (AQA A Level Biology) : Revision Note
The Haemoglobin Group
The haemoglobins are a group of chemically similar molecules that are found in many different organisms
For example chimpanzee, rats and humans are just some of the organisms that possess haemoglobin molecules
Structure
Haemoglobin is a globular protein which is an oxygen-carrying pigment found in vast quantities in red blood cells
Red blood cells are biconcave discs, meaning that they are concave on both sides
This creates a high SA:V ratio for the diffusion of gases
Red blood cells do not contain a nucleus
This provides more space inside the cell for haemoglobin so that they can transport as much oxygen as possible
Haemoglobin has a quaternary structure as it is made up of four polypeptide chains
These chains or subunits are globin proteins (two α–globins and two β–globins) and each subunit has a prosthetic haem group
The four globin subunits are held together by disulphide bonds and arranged so that their hydrophobic R groups are facing inwards, helping to preserve the three-dimensional spherical shape, and the hydrophilic R groups are facing outwards, helping to maintain solubility
The arrangements of the R groups is important to the functioning of haemoglobin; if changes occur to the sequence of amino acids in the subunits this can change the function of the protein, e.g.
In sickle cell anaemia a base substitution that results in the amino acid valine (non-polar) replacing glutamic acid (polar) makes haemoglobin less soluble
The prosthetic haem group contains an iron II ion (Fe2+) which is able to reversibly combine with an oxygen molecule, forming oxyhaemoglobin
The presence of oxyhaemoglobin causes blood to appear bright red in colour
Each haemoglobin with the four haem groups can therefore carry four oxygen molecules, or eight oxygen atoms
The haem group is the same for all types of haemoglobin but the globin chains can differ substantially between haemoglobins from different species
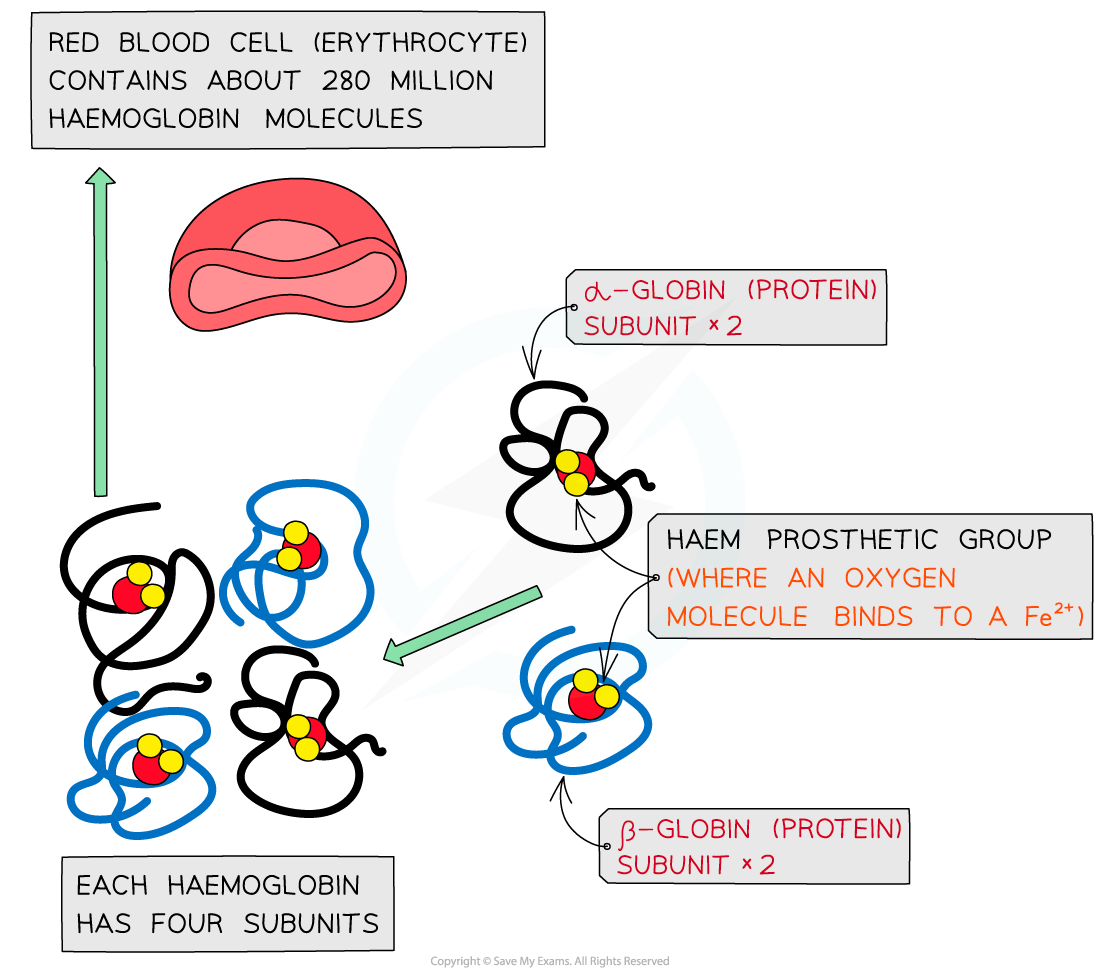
Haemoglobin contains α–globin and β–globin subunits and prosthetic haem groups which bind with oxygen molecules to form oxyhaemoglobin
Function
Haemoglobin is responsible for binding oxygen in the lungs and transporting the oxygen to the tissue to be used in aerobic metabolic pathways
As oxygen is not very soluble in water and haemoglobin is, oxygen can be carried more efficiently around the body when bound to the haemoglobin
The existence of the iron II ion (Fe2+) in the prosthetic haem group allows oxygen to reversibly bind
None of the amino acids that make up the polypeptide chains in haemoglobin are well suited to binding with oxygen
Examiner Tips and Tricks
You need to know the structure of haemoglobin and how this relates to its function, i.e. its ability to transport oxygen.

You've read 0 of your 5 free revision notes this week
Unlock more, it's free!
Did this page help you?

