Protein Structure & Function (AQA A Level Biology): Revision Note
Exam code: 7402
Proteins: structure & function
Structure
Proteins are macromolecules made from individual monomer units, amino acids
There are four levels of structure in proteins
Three are related to a single polypeptide chain
The fourth level relates to a protein that has two or more polypeptide chains
Protein molecules can have anywhere from three amino acids (Glutathione) to more than 34,000 amino acids (Titin) bonded together in chains
Primary structure
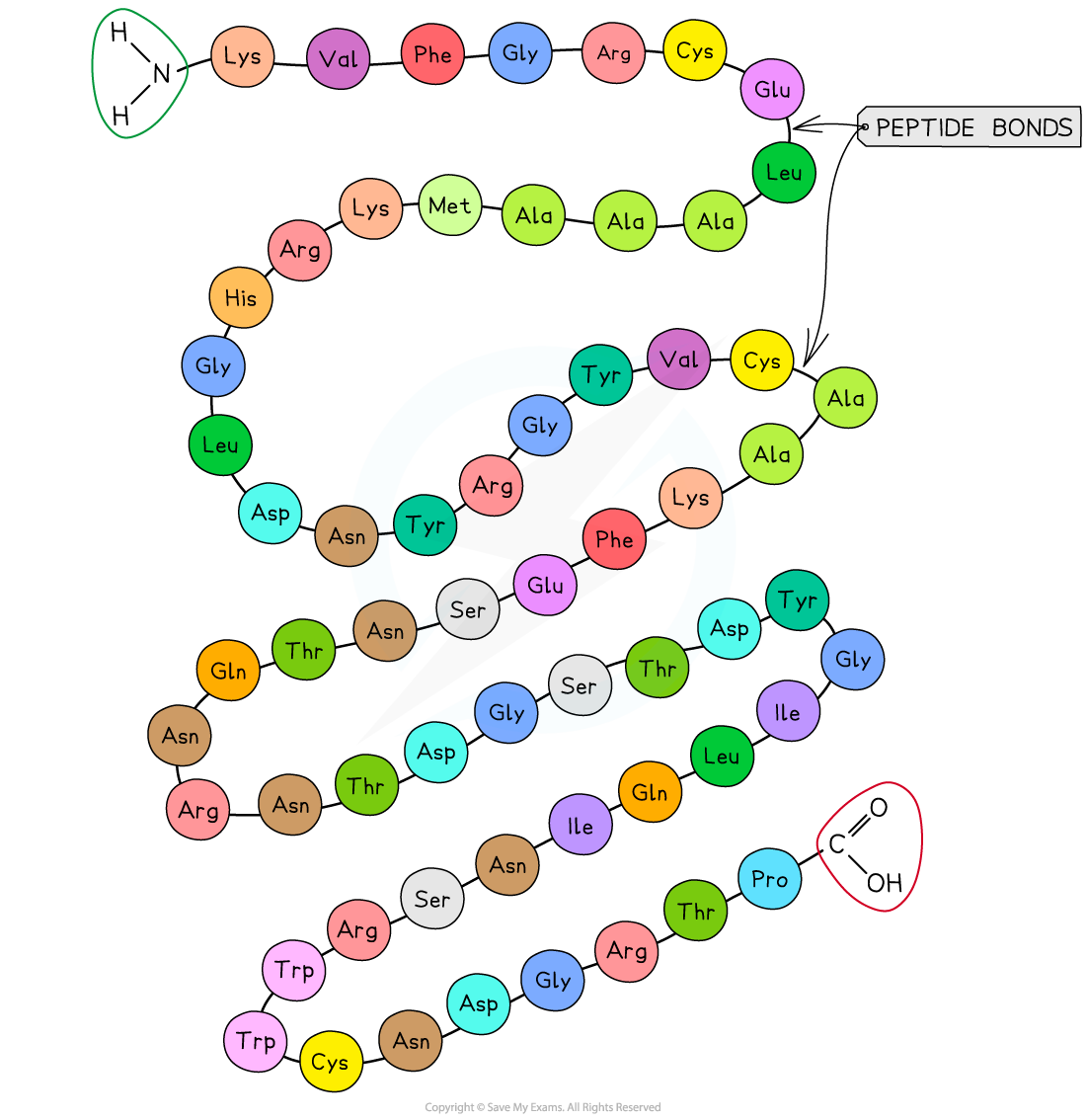
The sequence of amino acids bonded by peptide bonds is the primary structure of a protein
DNA of a cell determines the primary structure of a protein by instructing the cell to add certain amino acids in specific quantities in a certain sequence, during translation. This affects the shape and, therefore, the function of the protein
The primary structure is specific for each protein (one alteration in the sequence of amino acids can affect the function of the protein)
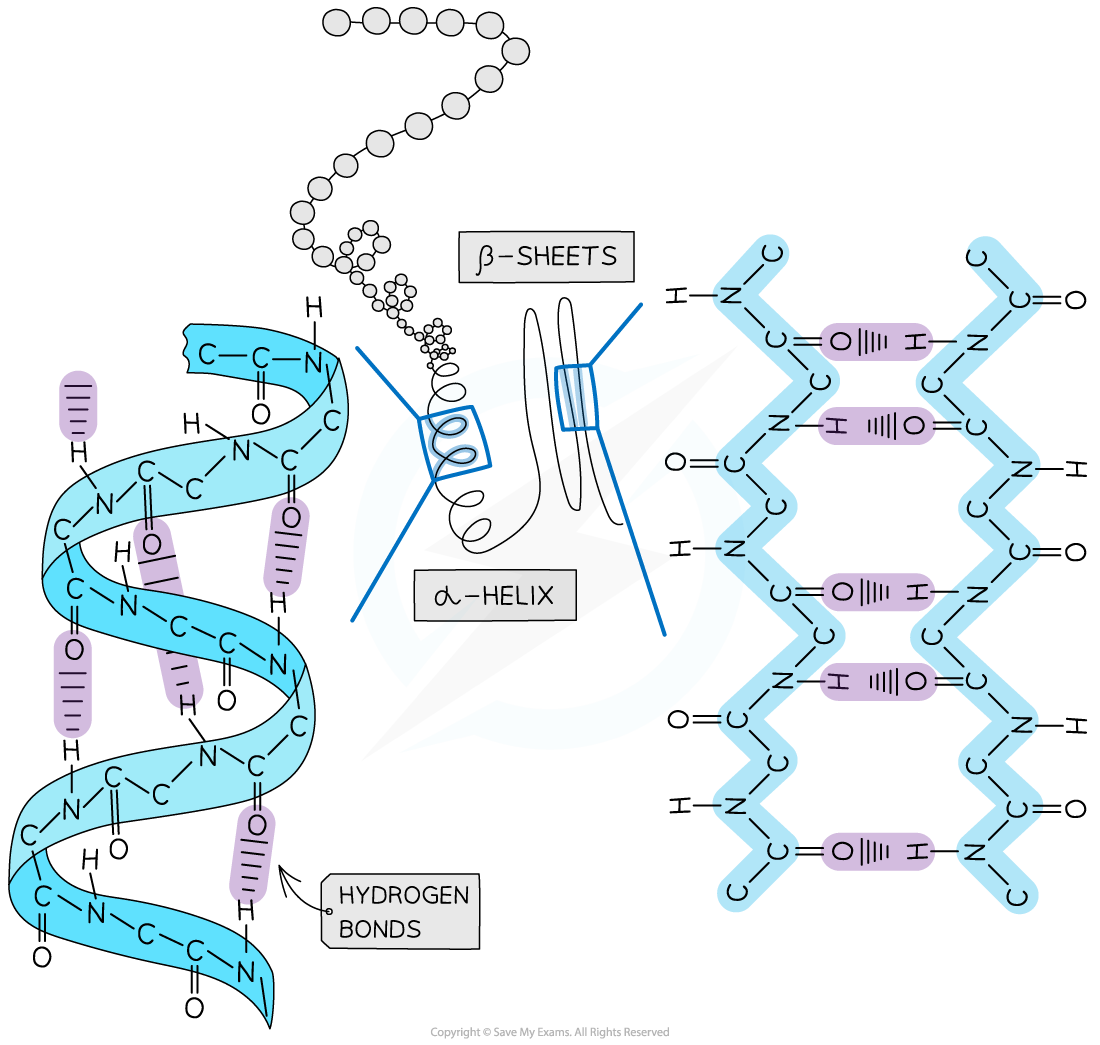
Secondary structure
The secondary structure of a protein is held together by hydrogen bonds that form between the -NH region of one amino acid and the -C=O region of another
The hydrogen of -NH has an overall positive charge, while the oxygen of -C=O has an overall negative charge
Hydrogen bonds are relatively weak, so they can be broken easily by high temperatures and pH changes
Two shapes can form within proteins due to the hydrogen bonds:
α-helix
β-pleated sheet
The α-helix shape occurs when the hydrogen bonds form between every fourth peptide bond
The β-pleated sheet shape forms when the protein folds so that two parts of the polypeptide chain are parallel to each other, enabling hydrogen bonds to form between the folded layers
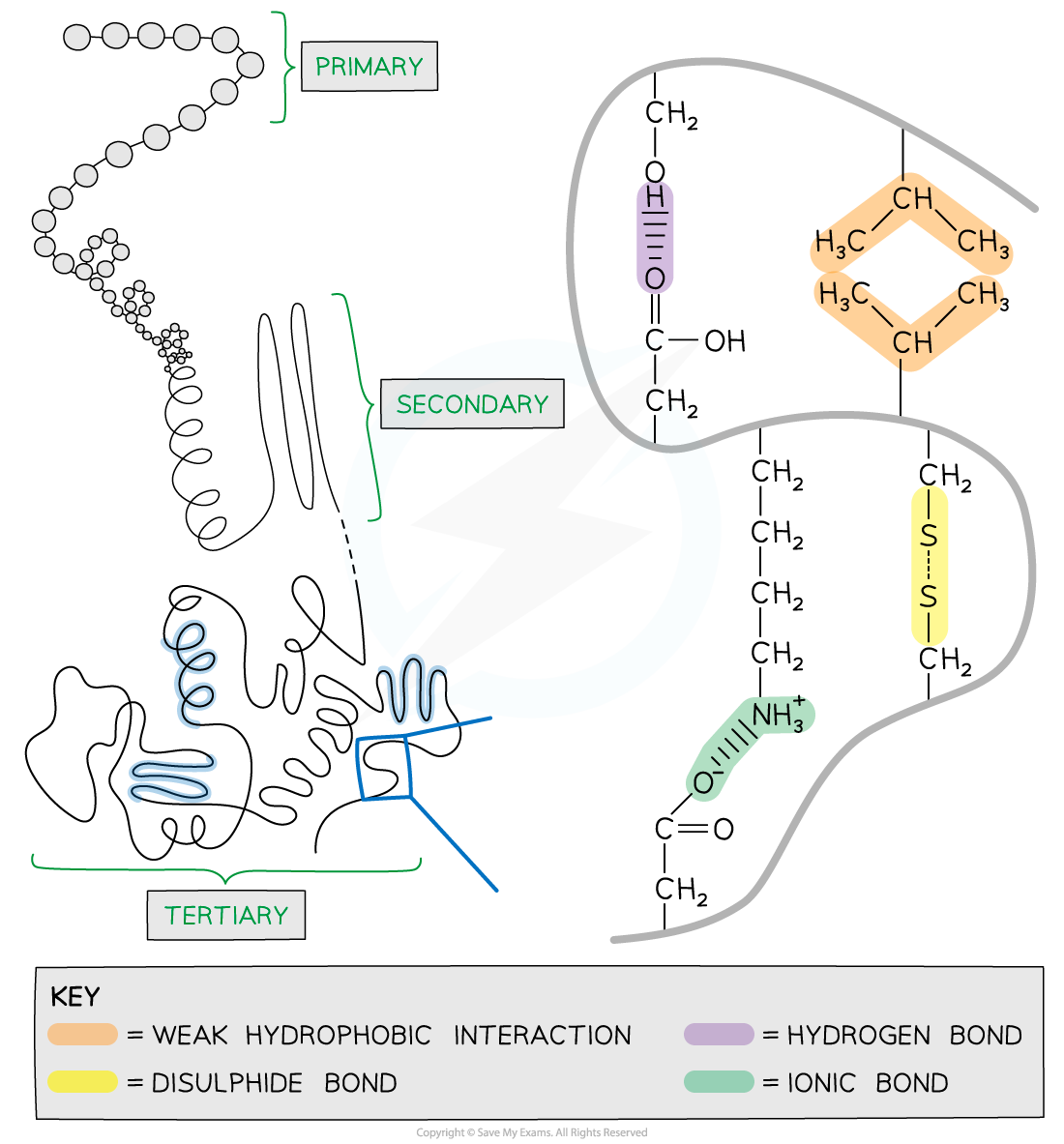
Tertiary structure
Further conformational change of the secondary structure leads to additional bonds forming between the R groups (side chains)
The additional bonds are:
hydrogen bonds between R groups
disulfide bonds between cysteine amino acids
ionic bonds between charged R groups
weak hydrophobic interactions between non-polar R groups
This structure is common in globular proteins such as enzymes and antibodies
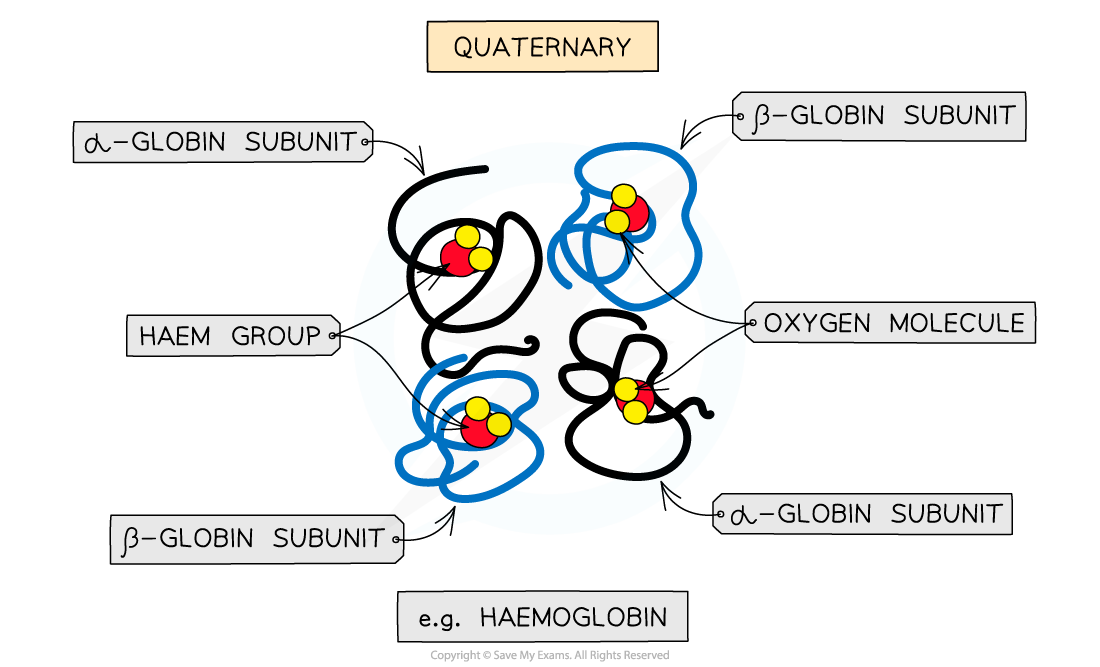
Quaternary structure
Occurs in proteins that have more than one polypeptide chain working together as a functional macromolecule, for example, haemoglobin
Each polypeptide chain in the quaternary structure is referred to as a subunit of the protein
Bond type | Structure of protein | ||
|---|---|---|---|
Primary | Secondary | Tertiary | |
Peptide | Yes | Yes | Yes |
Hydrogen | No | Yes - between amino and carboxyl groups | Yes - between R groups, and amino and carboxyl groups |
Disulphide | No | No | Yes |
Ionic | No | No | Yes |
Hydrophobic interactions | No | No | Yes |
Function
Proteins perform a wide range of essential roles in all living organisms due to their diverse structures
They are therefore vital for structure, transport, communication, defence, movement, and catalysis in all living cells:
Enzymes – biological catalysts that speed up metabolic reactions (e.g. amylase, DNA polymerase)
Transport proteins – carry substances (e.g. haemoglobin transports oxygen; channel proteins in membranes)
Structural proteins – provide support (e.g. collagen in connective tissues; keratin in hair and nails)
Hormones – regulate processes (e.g. insulin controls blood glucose levels)
Antibodies – part of the immune response, recognising and neutralising pathogens
Contractile proteins – enable movement (e.g. actin and myosin in muscles)
Examiner Tips and Tricks
Learn the four levels of protein structure and the types of bonds involved in each.
Remember that:
in secondary structure, hydrogen bonds form between the amino and carboxyl groups
in tertiary structure, hydrogen bonds form between the R groups of amino acids
You need to be able to relate the structure of proteins to the properties of proteins named throughout your specification, such as haemoglobin.

Unlock more, it's free!
Did this page help you?