The Glycosidic Bond (AQA A Level Biology): Revision Note
Exam code: 7402
Forming the glycosidic bond
To make monosaccharides more suitable for transport, storage and to have less influence on a cell’s osmolarity, they are bonded together to form disaccharides and polysaccharides
Disaccharides and polysaccharides are formed when two hydroxyl (-OH) groups (on different saccharides) interact to create a strong covalent bond called the glycosidic bond
Each glycosidic bond is catalysed by enzymes specific to which OH groups are interacting
Every glycosidic bond results in one water molecule being removed, thus, glycosidic bonds are formed by condensation
As there are many different monosaccharides, this results in different types of glycosidic bonds forming (e.g. maltose has an α-1,4 glycosidic bond and sucrose has an α-1,2 glycosidic bond)
Glycosidic bond in maltose
Maltose is a disaccharide formed by the condensation reaction of two glucose molecules

Glycosidic bond in sucrose
Sucrose is a disaccharide formed by the condensation of a glucose molecule and a fructose molecule

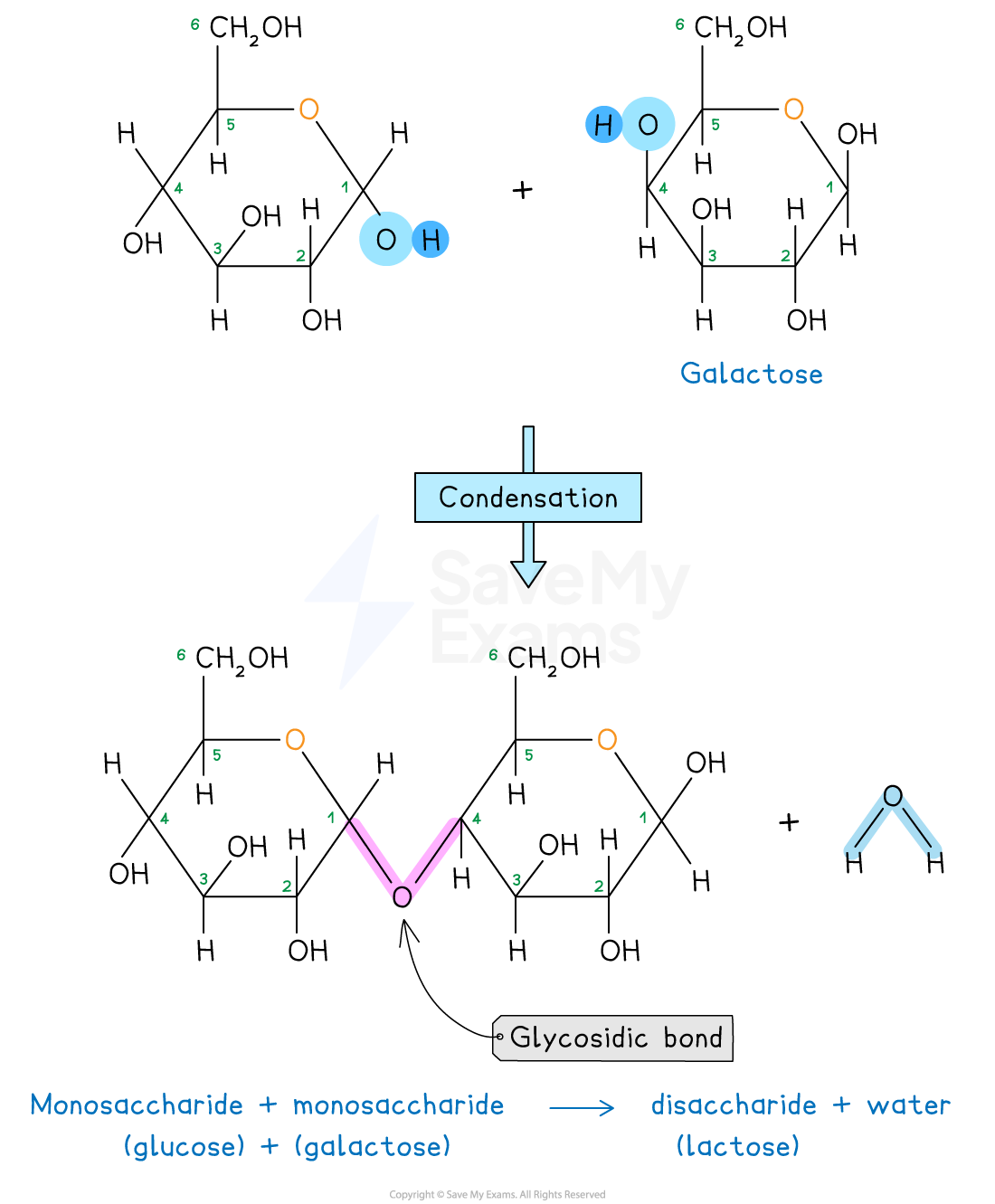
Glycosidic bond in lactose
Lactose is a disaccharide formed by the condensation of a glucose molecule and a galactose molecule
Glycosidic bond in a polysaccharide
Polysaccharides are formed by the condensation of many glucose units
E.g. Glycogen and starch are formed by the condensation of α-glucose
E.g. Cellulose is formed by the condensation of β-glucose

Examiner Tips and Tricks
Make sure you can identify where the glycosidic bond is in a carbohydrate. You need to know the specific examples discussed on this page.
Breaking the glycosidic bond
The glycosidic bond is broken when water is added in a hydrolysis (meaning ‘hydro’ - with water and ‘lyse’ - to break) reaction
Hydrolytic reactions are catalysed by enzymes, these are different to those present in condensation reactions
Disaccharides and polysaccharides are broken down into smaller molecules in hydrolysis reactions
Examples of hydrolytic reactions include the digestion of food in the alimentary tract and the breakdown of stored carbohydrates in muscle and liver cells for use in cellular respiration

Examiner Tips and Tricks
Remember that disaccharides hydrolyse to two monosaccharides, whereas polysaccharides must undergo many hydrolytic reactions until they form monosaccharides.

Unlock more, it's free!
Did this page help you?